Coprecipitation of Ce(III) oxide with UO2
- PMID: 39347702
- PMCID: PMC11542660
- DOI: 10.1107/S1600577524008336
Coprecipitation of Ce(III) oxide with UO2
Abstract
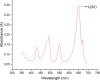
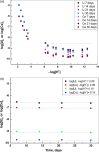
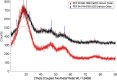
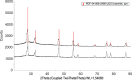
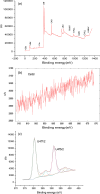
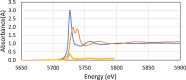
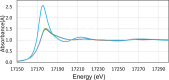
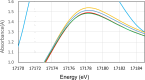
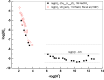
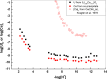
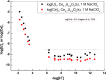
The neutralization of acidic solutions containing U (IV) and Ce (III) at room temperature in glove box atmosphere and in the presence of dithionite results in coprecipitation of these elements as amorphous solid solutions CexU1-xO2±y. The solubilities of the precipitates with different mole fractions (x) of Ce(OH)3 (x = 0.01 or 0.1) were determined in 1 M NaClO4 solutions between pH 2.2 and 12.8 under reducing conditions. The solids were investigated by a variety of methods (chemical analysis, SEM-EDX, XRD, XPS, XAS) to determine the nature of the solid solutions formed, their composition and the valence state of Ce and U. X-ray photoelectron spectroscopy confirmed the oxidation states of the solids both before and after the equilibration as Ce (III) and U (IV). The amorphous coprecipitates reached equilibrium relatively fast (∼1 week). The release of Ce from the coprecipitates was totally dominated by the release of uranium over the whole pH range. The Ce concentrations decrease slightly with the decrease of Ce content in the solid, suggesting that CexU1-xO2±y solids behave thermodynamically as solid solutions. The concentrations of U in equilibrium with the coprecipitate were in excellent agreement with the solubility of UO2(s) under reducing conditions reported in the literature. The conditional solubility product of Ce(OH)3 from the coprecipitate was several orders of magnitude (∼4 in the near neutral pH range and ∼18 in the acidic range) lower than that of pure Ce(OH)3(s). The activities and activity coefficients of Ce(OH)3(s) in the coprecipitate were also estimated. Activity coefficients are much less than 1, indicating that the mixing of Ce(OH)3 with UO2 is highly favorable.
Keywords: Ce (III); UO2; actinides; amorphous; coprecipitation; solid solution; solubility.
open access.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Allen, P. G., Shuh, D. K., Bucher, J. J., Edelstein, N. M., Palmer, C. E. A. & Marquez, L. N. (1996). EXAFS Spectroscopic Study of Uranium (VI) Precipitates, Report LBNL-39934. Ernest Orlando Lawrence Berkeley National Laboratory, CA USA.
-
- Arab-Chapelet, B., De Bruycker, F., Picart, S., Leturcq, G. & Grandjean, S. (2008). Proceedings of ATALANTE 2008: Nuclear Fuel Cycles for a Sustainable Future, 19–23 May 2008, Montpellier, France. Paper 3,02.
-
- Baes, C. F. & Mesmer, R. E. (1976). The Hydrolysis of Cations. New York: Wiley.
-
- Bernkopf, M. F. (1984). Hydrolysereaktionen und karbonatkomplexierung von dreiwertigem americium im Natürlichen aquatischen systemen. Thesis. Technische Universität München, Germany.
-
- Broczkowski, M., Noël, J. & Shoesmith, D. (2005). J. Nucl. Mater.346, 16–23.
Grants and funding
LinkOut - more resources
Full Text Sources

