Development and characterization of formulations based on combinatorial potential of antivirals against genital herpes
- PMID: 39347802
- PMCID: PMC11919951
- DOI: 10.1007/s00210-024-03468-y
Development and characterization of formulations based on combinatorial potential of antivirals against genital herpes
Erratum in
-
Correction: Development and characterization of formulations based on combinatorial potential of antivirals against genital herpes.Naunyn Schmiedebergs Arch Pharmacol. 2025 Mar;398(3):3179. doi: 10.1007/s00210-024-03668-6. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39636407 Free PMC article. No abstract available.
Abstract
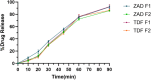
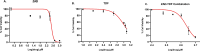
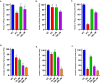
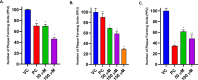
Herpes simplex virus type 2 (HSV-2) treatment faces challenges due to antiviral resistance and systemic side effects of oral therapies. Local delivery of antiviral agents, such as tenofovir (TDF) and zinc acetate dihydrate (ZAD), may offer improved efficacy and reduced systemic toxicity. This study's objective is to develop and evaluate local unit dose formulations of TDF and ZAD combination for local treatment of HSV-2 infection and exploring their individual and combinatory effects in vitro. The study involved the development of immediate-release film and pessary formulations containing TDF and ZAD. These formulations were characterized for physicochemical properties and in vitro drug release profiles. Cytotoxicity and antiviral activity assays were conducted to evaluate the individual and combinatory effects of TDF and ZAD. Film formulations released over 90% of the drugs within 1 h, and pessary formulations within 90 min, ensuring effective local drug delivery. ZAD showed moderate antiviral activity while TDF exhibited significant antiviral activity at non-cytotoxic concentrations. The combination of TDF and ZAD demonstrated synergistic effects in co-infection treatments, reducing the concentration required for 50% inhibition of HSV-2. Developed film and pessary formulations offer consistent and predictable local drug delivery, enhancing antiviral efficacy while minimizing systemic side effects. The combination of TDF and ZAD showed potential synergy against HSV-2, particularly in co-infection treatments. Further preclinical studies on pharmacokinetics, safety, and efficacy are necessary to advance these formulations toward clinical application.
Keywords: Antivirals; Combination therapy; Formulations; Genital herpes; Good health and well-being; Local drug delivery.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Human ethics and consent to participate: NA Competing interests: The authors declare no competing interests.
Figures


References
-
- Apriliyani MW, Purwadi Manab A, Ikhwan AD (2020) Characteristics of moisture content, swelling, opacity and transparency with addition chitosan as edible films/coating base on casein. Adv J Food Sci Technol 18:9–14. 10.19026/ajfst.18.6041
-
- Avlani D, Kumar A, HN S (2023) Development of dispersible vaginal tablets of tenofovir loaded mucoadhesive chitosan microparticles for anti-HIV pre-exposure prophylaxis. Mol Pharmaceutics 20:5006–5018. 10.1021/acs.molpharmaceut.3c00288 - PubMed
-
- Avlani D, Shivakumar HN, Kumar A et al (2024) Pre-exposure prophylactic mucoadhesive sodium alginate microsphere laden pessaries for intravaginal delivery of tenofovir disoproxil fumarate. Int J Biol Macromol 258:128816. 10.1016/j.ijbiomac.2023.128816 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

