12-Month Time in Tight Range Improvement with Advanced Hybrid-Closed Loop System in Adults with Type 1 Diabetes
- PMID: 39347899
- PMCID: PMC11561210
- DOI: 10.1007/s13300-024-01656-w
12-Month Time in Tight Range Improvement with Advanced Hybrid-Closed Loop System in Adults with Type 1 Diabetes
Abstract
Introduction: Time in tight range (TITR) is an emerging and valuable metric for assessing normoglycemia. The latest advancement in automated insulin delivery (AID) systems, the advanced hybrid closed-loop (AHCL) systems, are particularly noteworthy for managing type 1 diabetes (T1D) and enhancing glycemic control.
Methods: In a real-world clinical setting, we carried out a retrospective evaluation of TITR in 42 adult subjects with T1D using the AHCL Minimed™ 780G system over a 12-month period.
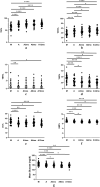
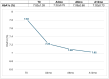
Results: Within just 14 days of activating the automatic mode, the AHCL Minimed™ 780G system showed rapid improvement in TITR, and in the other continuous glucose monitoring (CGM) metrics. This improvement persisted over 12 months, achieving the proposed 45-50% range for effective glycemic control.
Conclusion: The AHCL Minimed™ 780G system significantly enhances TITR, demonstrating continuous improvement throughout a 12-month follow-up period.
Keywords: Advanced hybrid closed-loop systems; Continuous glucose monitoring metrics; Technology; Time in tight range; Type 1 diabetes.
© 2024. The Author(s).
Conflict of interest statement
Figures

References
LinkOut - more resources
Full Text Sources

