Time Course of Reversal of Fentanyl-Induced Respiratory Depression in Healthy Subjects by Intramuscular Nalmefene and Intramuscular and Intranasal Naloxone
- PMID: 39347921
- PMCID: PMC11771590
- DOI: 10.1002/jcph.6132
Time Course of Reversal of Fentanyl-Induced Respiratory Depression in Healthy Subjects by Intramuscular Nalmefene and Intramuscular and Intranasal Naloxone
Abstract
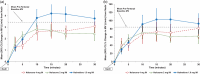
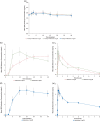
The increase in opioid overdose deaths, particularly involving potent, long-acting synthetic opioids, has led to calls for stronger, longer-acting opioid-overdose-reversal agents. Using an opioid-induced respiratory depression model, we investigated the onset and time course of action of naloxone and a long-acting opioid antagonist, nalmefene, in reversing the effects of an ongoing intravenous fentanyl infusion over a period of up to 100 min. Healthy, moderately experienced opioid users received intramuscular (IM) nalmefene 1 mg, IM naloxone 2 mg, or intranasal (IN) naloxone 4 mg after fentanyl-induced respiratory depression was established based on reduction in respiratory minute volume (MV). Each participant received each opioid antagonist twice per a randomized crossover schedule. Reversal of respiratory depression, pharmacokinetics, and safety were investigated. Participants showed rapid increases in plasma opioid antagonist concentrations, and meaningful reversal of depressed MV tended to occur earlier with IM nalmefene and IM naloxone than with IN naloxone. Compared to naloxone, nalmefene provided extended exposure, and mean MV was maintained at a higher level. All participants experienced treatment-related adverse events, but none were severe, serious, or led to study drug discontinuation. This study provides evidence that IM nalmefene 1 mg achieves reversal of fentanyl-induced respiratory depression similar to or better than that achieved with standard-of-care naloxone treatments. No new safety concerns were raised for IM nalmefene at the tested dose. The pharmacokinetic and pharmacodynamic properties of IM nalmefene position it as an important treatment option in opioid overdose reversal, particularly given the increasing prevalence of overdoses involving potent, long-acting synthetic opioids.
Keywords: fentanyl; nalmefene; naloxone; opioid antagonist; opioid overdose; pharmacodynamics; pharmacokinetics; respiratory depression reversal.
© 2024 The Author(s). The Journal of Clinical Pharmacology published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
AC, EH, MS, and SCH are employees of Imbrium Therapeutics L.P., a subsidiary of Purdue Pharma L.P., the study sponsor. RPK was an employee of Imbrium Therapeutics L.P. at the time of the study. GA received no direct compensation for work related to the development of this manuscript. GA was directly compensated by Purdue Pharma L.P. for his involvement as the principal investigator for this study. SCH owns stock/stock options in Pfizer.
Figures


References
-
- Ahmad FB, Rossen LM, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics. 2021. Accessed March 28, 2022. https://www.cdc.gov/nchs/nvss/vsrr/drug‐overdose‐data.htm
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

