Sustained co-release of ciprofloxacin and dexamethasone in rabbit maxillary sinus using polyvinyl alcohol-based hydrogel microparticle
- PMID: 39348071
- PMCID: PMC11442669
- DOI: 10.1007/s10856-024-06832-9
Sustained co-release of ciprofloxacin and dexamethasone in rabbit maxillary sinus using polyvinyl alcohol-based hydrogel microparticle
Abstract
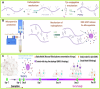
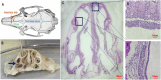
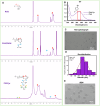
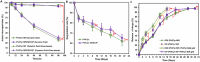
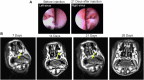
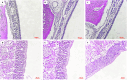
Topical delivery to paranasal sinuses through sustained-release stents is one of the new horizons in treating chronic rhinosinusitis (CRS). This study aims to introduce and evaluate sustained co-release of encapsulated ciprofloxacin (CIP) and dexamethasone (DEX) in polyvinyl alcohol-based carriers within the maxillary sinus of rabbit animals. DEX and CIP were loaded in a tyramine-substituted polyvinyl alcohol microparticle (PVATyr MP). The mechanical stability, degradability, and sustained-release patterns of both drugs as well as cellular cytocompatibility were assessed in vitro. The PVATyr MPs were then injected into the maxillary sinus of rabbits and they were monitored weekly for 21 days. Nasal endoscopy, MRI imaging, and tissue microscopy were used to follow the changes and compared them with the control condition. Also, the concentrations of drugs were evaluated in the maxillary sinus and blood samples over the study period. Produced PVA-based MPs possessed a relatively narrow particle size distribution (CV 7.7%) with proper physical stability until 30 days of incubation. The uniform-sized PVATyr MPs and their surrounding hydrogel showed sustained-release profiles for DEX and CIP for up to 32 days in vitro. The injected drugs-loaded hydrogel showed complete clearance from the maxillary sinus of rabbits within 28 days. The concentrations of DEX and CIP in mucosal remained within the therapeutic window when measured on days 7, 14, and 21, which were well above the plasma concentrations without any pathological changes in endoscopy, MRI imaging, and histological examinations. DEX/CIP loaded PVATyr MPs provided an effective, controlled, and safe sustained-drug delivery in both in vitro and in vivo analyses at therapeutic concentrations with minimal systemic absorption, suggesting a promising treatment approach for CRS.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

