Ticagrelor and Statins: Dangerous Liaisons?
- PMID: 39348077
- PMCID: PMC11680608
- DOI: 10.1007/s10557-024-07624-7
Ticagrelor and Statins: Dangerous Liaisons?
Abstract
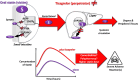
Polypharmacy is often necessary in complex, chronic, comorbid and cardiovascular patients and is a known risk factor for potential drug-drug interaction (DDI) that can cause adverse reactions (toxicity or therapeutic failure). Anti-thrombotic drugs (largely low-dose aspirin and a platelet P2Y12 receptor inhibitor) and statins are among the most co-administered drugs in cardiovascular patients. Ticagrelor is a selective antagonist of the platelet P2Y12-receptor, highly effective in inhibiting platelet aggregation and bio-transformed by the CYP3A4 and substrate of transporters, such as the breast cancer resistance protein (BCRP). Statins have different pharmacokinetic profiles; some undergo CYP3A4-mediated metabolism; rosuvastatin is primarily metabolized by the CYP2C9; and they have different affinities for drug transporters. Rhabdomyolysis is a very rare but severe adverse event, which is specific for statins which can be triggered by DDIs that increase statin's concentrations through blockade of their biotransformation and/or elimination. Large pharmacovigilance and small observational studies reported increased rhabdomyolysis in patients treated with some statins and ticagrelor but not aspirin, clopidogrel or prasugrel. Recent studies in vitro, pharmacokinetic trials and in silico drug modelling identified and validated the BCRP inhibition by ticagrelor, as a mechanism contributing to the DDI with statins, as 'victim' drugs, leading to increased rhabdomyolysis. While the clinical impact of this DDI deserves further investigation, a careful evaluation should be advised when ticagrelor is co-prescribed with some statins.
Keywords: Cytochrome P450; Drug interactions; Rhabdomyolysis; Statins; Ticagrelor; Transporters.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: Not relevant for an editorial. Consent to Participate: Not relevant for an editorial. Consent for Publication: Not relevant for an editorial. Competing Interests: The authors declare no competing interests.
Figures
Similar articles
-
Rosuvastatin-Induced Rhabdomyolysis - Possible Role of Ticagrelor and Patients' Pharmacogenetic Profile.Basic Clin Pharmacol Toxicol. 2018 Oct;123(4):509-518. doi: 10.1111/bcpt.13035. Epub 2018 Jun 29. Basic Clin Pharmacol Toxicol. 2018. PMID: 29734517 Review.
-
Efficacy of P2Y12 Receptor Blockers After Myocardial Infarction and Genetic Variability of their Metabolic Pathways.Curr Vasc Pharmacol. 2019;17(1):35-40. doi: 10.2174/1570161116666180206110657. Curr Vasc Pharmacol. 2019. PMID: 29412111 Review.
-
Pharmacology of the new P2Y12 receptor inhibitors: insights on pharmacokinetic and pharmacodynamic properties.Drugs. 2013 Oct;73(15):1681-709. doi: 10.1007/s40265-013-0126-z. Drugs. 2013. PMID: 24114622 Review.
-
Pharmacokinetic interaction studies of co-administration of ticagrelor and atorvastatin or simvastatin in healthy volunteers.Eur J Clin Pharmacol. 2013 Mar;69(3):477-87. doi: 10.1007/s00228-012-1369-4. Epub 2012 Aug 25. Eur J Clin Pharmacol. 2013. PMID: 22922682 Clinical Trial.
-
Assessment of the Risk of Rhabdomyolysis and Myopathy During Concomitant Treatment with Ticagrelor and Statins.Drugs. 2018 Jul;78(11):1105-1112. doi: 10.1007/s40265-018-0947-x. Drugs. 2018. PMID: 30003466 Free PMC article. Review.
Cited by
-
Ticagrelor is Associated with Increased Rosuvastatin Blood Concentrations in Patients who have had a Myocardial Infarction.Clin Pharmacokinet. 2025 Apr;64(4):565-571. doi: 10.1007/s40262-025-01489-1. Epub 2025 Mar 3. Clin Pharmacokinet. 2025. PMID: 40029501 Free PMC article.
-
Author's Reply to Chan et al.: "Ticagrelor is Associated with Increased Rosuvastatin Blood Concentrations in Patients Who Have Had a Myocardial Infarction".Clin Pharmacokinet. 2025 Jul;64(7):1137-1138. doi: 10.1007/s40262-025-01519-y. Epub 2025 May 23. Clin Pharmacokinet. 2025. PMID: 40408048 No abstract available.
-
A Comprehensive Review of the Latest Approaches to Managing Hypercholesterolemia: A Comparative Analysis of Conventional and Novel Treatments: Part I.Life (Basel). 2025 Jul 25;15(8):1185. doi: 10.3390/life15081185. Life (Basel). 2025. PMID: 40868833 Free PMC article. Review.
-
Oral P2Y12 Inhibitors: Victims or Perpetrators? A Focused Review on Pharmacokinetic, Clinically Relevant Drug Interactions.Eur Cardiol. 2025 Jun 11;20:e17. doi: 10.15420/ecr.2025.12. eCollection 2025. Eur Cardiol. 2025. PMID: 40556646 Free PMC article. Review.
References
-
- Tamargo J, Kjeldsen KP, Delpon E, et al. Facing the challenge of polypharmacy when prescribing for older people with cardiovascular disease. A review by the European society of cardiology working group on cardiovascular pharmacotherapy. Eur Heart J Cardiovasc Pharmacother. 2022;8(4):406–19. 10.1093/ehjcvp/pvac005. - PubMed
-
- Sahoo AK, Singh A, Gupta D, Dhaneria S, Arunima P. Assessment of potential drug-drug Interactions (pDDIs) and their risk factors among hospitalized cardiac patients in a tertiary-care center of Central India: a retrospective record-based study. Hosp Pharm. 2024;59(1):24–31. 10.1177/00185787231182569. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

