Reactive Passivation of Wide-Bandgap Organic-Inorganic Perovskites with Benzylamine
- PMID: 39348291
- PMCID: PMC11467896
- DOI: 10.1021/jacs.4c06659
Reactive Passivation of Wide-Bandgap Organic-Inorganic Perovskites with Benzylamine
Abstract
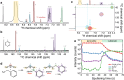
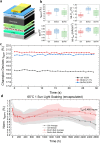
While amines are widely used as additives in metal-halide perovskites, our understanding of the way amines in perovskite precursor solutions impact the resultant perovskite film is still limited. In this paper, we explore the multiple effects of benzylamine (BnAm), also referred to as phenylmethylamine, used to passivate both FA0.75Cs0.25Pb(I0.8Br0.2)3 and FA0.8Cs0.2PbI3 perovskite compositions. We show that, unlike benzylammonium (BnA+) halide salts, BnAm reacts rapidly with the formamidinium (FA+) cation, forming new chemical products in solution and these products passivate the perovskite crystal domains when processed into a thin film. In addition, when BnAm is used as a bulk additive, the average perovskite solar cell maximum power point tracked efficiency (for 30 s) increased to 19.3% compared to the control devices 16.8% for a 1.68 eV perovskite. Under combined full spectrum simulated sunlight and 65 °C temperature, the devices maintained a better T80 stability of close to 2500 h while the control devices have T80 stabilities of <100 h. We obtained similar results when presynthesizing the product BnFAI and adding it directly into the perovskite precursor solution. These findings highlight the mechanistic differences between amine and ammonium salt passivation, enabling the rational design of molecular strategies to improve the material quality and device performance of metal-halide perovskites.
Conflict of interest statement
The authors declare the following competing financial interest(s): H.J.S. is co-founder and CSO of Oxford PV Ltd.
Figures


References
-
- Schmidt-Mende L.; Dyakonov V.; Olthof S.; Ünlü F.; Lê K. M. T.; Mathur S.; Karabanov A. D.; Lupascu D. C.; Herz L. M.; Hinderhofer A.; Schreiber F.; Chernikov A.; Egger D. A.; Shargaieva O.; Cocchi C.; Unger E.; Saliba M.; Byranvand M. M.; Kroll M.; Nehm F.; Leo K.; Redinger A.; Höcker J.; Kirchartz T.; Warby J.; Gutierrez-Partida E.; Neher D.; Stolterfoht M.; Würfel U.; Unmüssig M.; Herterich J.; Baretzky C.; Mohanraj J.; Thelakkat M.; Maheu C.; Jaegermann W.; Mayer T.; Rieger J.; Fauster T.; Niesner D.; Yang F.; Albrecht S.; Riedl T.; Fakharuddin A.; Vasilopoulou M.; Vaynzof Y.; Moia D.; Maier J.; Franckevičius M.; Gulbinas V.; Kerner R. A.; Zhao L.; Rand B. P.; Glück N.; Bein T.; Matteocci F.; Castriotta L. A.; Di Carlo A.; Scheffler M.; Draxl C. Roadmap on Organic-Inorganic Hybrid Perovskite Semiconductors and Devices. APL Mater. 2021, 9 (10), 109202. 10.1063/5.0047616. - DOI
-
- Best Research-Cell Efficiency Chart | Photovoltaic Research | NREL, 2023. https://www.nrel.gov/pv/cell-efficiency.html (accessed 2023-09-29).
-
- Hörantner M. T.; Snaith H. J. Predicting and Optimising the Energy Yield of Perovskite-on-Silicon Tandem Solar Cells under Real World Conditions. Energy Environ. Sci. 2017, 10 (9), 1983–1993. 10.1039/C7EE01232B. - DOI

