Cytotoxicity of bendamustine, alone and in combination with novel agents, toward adult T-cell leukemia cells
- PMID: 39348376
- PMCID: PMC11441677
- DOI: 10.1371/journal.pone.0309533
Cytotoxicity of bendamustine, alone and in combination with novel agents, toward adult T-cell leukemia cells
Abstract
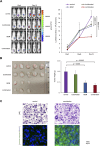
Adult T-cell leukemia/lymphoma (ATL) develops from the infection of T cells with human T lymphotropic virus type 1 (HTLV-1). There are an estimated 5-20 million HTLV-1 carriers worldwide and the patients are frequently observed in subtropical Africa, the Caribbean, Middle East, South America, and South West Japan. The prognosis of ATL remains dismal due to rapid acquired resistance to treatment with cytotoxic chemotherapeutic agents. In particular, the development of novel therapies for relapsed or refractory (R/R) ATL is an unmet need. Previous clinical trials revealed that bendamustine (BDM) was effective as the first-line treatment for indolent lymphoma and R/R cases of diffuse large B-cell lymphoma. Its major advantage is that it has few side effects such as hair loss and peripheral neuropathy, and does not impair the quality of life. However, its efficacy has not been verified for ATL in pre-clinical or clinical studies. In this study, we have shown the cytotoxicity of BDM alone and in combination with novel agents including the histone deacetylase (HDAC) inhibitor tucidinostat, the enhancer of zeste homolog 1/2 (EZH1/2) dual inhibitor valemetostat, and the Bcl2 family inhibitor ABT-737. The combined in vitro effects of BDM and tucidinostat were reproduced in a murine model without any obvious hematological toxicity. Our present results suggest that the combination of tucidinostat and BDM could additively prolong the survival of patients with R/R progressive ATL. The efficacy and safety of this combination are thus worthy of investigation in clinical settings.
Copyright: © 2024 Osada et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Yusuke Furukawa received research funding from SymBio Pharmaceuticals Ltd. This does not alter our adherence to PLOS ONE policies on sharing data and materials. Other authors have no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

