Omission of Chemoradiation in Locally Advanced Rectal Adenocarcinoma: Evaluation of PROSPECT in a National Database
- PMID: 39348440
- PMCID: PMC11849708
- DOI: 10.1002/jso.27839
Omission of Chemoradiation in Locally Advanced Rectal Adenocarcinoma: Evaluation of PROSPECT in a National Database
Erratum in
-
Correction to "Omission of Chemoradiation in Locally Advanced Rectal Adenocarcinoma: Evaluation of PROSPECT in a National Database".J Surg Oncol. 2025 Dec;132(8):1422. doi: 10.1002/jso.70125. Epub 2025 Nov 4. J Surg Oncol. 2025. PMID: 41186048 No abstract available.
Abstract
Background and objectives: The PROSPECT trial showed noninferiority of neoadjuvant chemotherapy (NAC) with selective chemoradiation (CRT) versus CRT alone. However, trial results are often difficult to reproduce with real-world data. Pathologic outcomes and overall survival (OS) were evaluated by neoadjuvant strategy in locally advanced rectal adenocarcinoma patients in a national database.
Methods: The 2012-2020 National Cancer Database was queried for clinical T2N1 and T3N0-1 rectal adenocarcinoma patients with definitive resection. Patients were categorized by neoadjuvant treatment with CRT alone, NAC alone, and NAC with CRT. Outcomes included R0 resection, pathologic complete response (PCR), and OS.
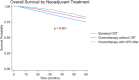
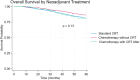
Results: Of 18 892 patients, 16 126 (85.4%) received CRT, 1018 (5.4%) NAC, and 1748 (9.3%) NAC with CRT. Patients with NAC alone or NAC with CRT were more likely to have stage-III disease, private insurance, and academic facility treatment (all p < 0.001). NAC alone had lower adjusted odds of an R0 resection (OR 0.72; 95%CI 0.54-0.95) and PCR (OR 0.77; 95%CI 0.64-0.93). NAC with CRT demonstrated improved OS (HR 0.71; 95%CI 0.61-0.82), with no difference between NAC and CRT alone. Among patients who received adjuvant chemotherapy, no differences in OS were seen.
Conclusions: Patients who received NAC alone had worse pathologic outcomes. NAC had similar OS to CRT and NAC with CRT showed improved OS.
Keywords: chemoradiation; locally advanced rectal adenocarcinoma; neoadjuvant therapy; overall survival; pathologic complete response.
© 2024 The Author(s). Journal of Surgical Oncology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Peeters K. C. M. J., Marijnen C. A. M., Nagtegaal I. D., et al., “The TME Trial After a Median Follow‐Up of 6 Years: Increased Local Control but no Survival Benefit in Irradiated Patients With Resectable Rectal Carcinoma,” Annals of Surgery 246, no. 5 (November 2007): 693–701, 10.1097/01.sla.0000257358.56863.ce. - DOI - PubMed
MeSH terms
Grants and funding
- R38 CA245095/CA/NCI NIH HHS/United States
- T32 CA247801/CA/NCI NIH HHS/United States
- Joanna T. Buchheit was funded by the National Institutes of Health's training grant 5R38CA245095; Surgical Multispecialty Access to Research in Residency Training (SMART) at Northwestern University. Lauren M. Janczewski is supported by a training grant from the National Cancer Institute (T32CA247801).
LinkOut - more resources
Full Text Sources
Research Materials

