What we know so far and what we can expect next: A molecular investigation of plant parasitism
- PMID: 39348487
- PMCID: PMC11441458
- DOI: 10.1590/1678-4685-GMB-2024-0051
What we know so far and what we can expect next: A molecular investigation of plant parasitism
Abstract
The review explores parasitic plants' evolutionary success and adaptability, highlighting their widespread occurrence and emphasizing the role of an invasive organ called haustorium in nutrient acquisition from hosts. It discusses the genetic and physiological adaptations that facilitate parasitism, including horizontal gene transfer, and the impact of environmental factors like climate change on these relationships. It addresses the need for further research into parasitic plants' genomes and interactions with their hosts to better predict environmental changes' impacts.
Conflict of interest statement
Figures
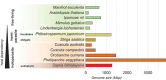
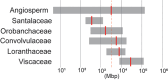


References
-
- Ackroyd RD, Graves JD. Sorghum bicolor and Striga hermonthica. Vol. 80. Ann Bot; 1997. The regulation of the water potential gradient in the host and parasite relationship between; pp. 649–656.
-
- Amutenya A, Kwembeya E, Shikangalah R, Tsvuura Z. Photosynthesis, chlorophyll content and water potential of a mistletoe-host pair in a semi-arid savanna. S Afr J Bot. 2023;163:311–315.
Internet Resources
-
- Leitch IJ, Johnston E, Pellicer J, Hidalgo O, Bennett MD. Plant DNA C-values Database. [1, Apr 2019];Plant DNA C-values database. 2019 https://cvalues.science.kew.org/search . accessed 25 May 2024.
-
- U.S. DEPARTMENT OF AGRICULTURE . Parasitic Plant Genera List. 2024. https://www.aphis.usda.gov/organism-soil-import/federal-noxious-weeds/pa... accessed 25 May 2024.
LinkOut - more resources
Full Text Sources

