PBBM Considerations for Base Models, Model Validation, and Application Steps: Workshop Summary Report
- PMID: 39348508
- PMCID: PMC11539057
- DOI: 10.1021/acs.molpharmaceut.4c00758
PBBM Considerations for Base Models, Model Validation, and Application Steps: Workshop Summary Report
Abstract
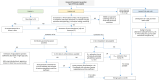
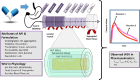
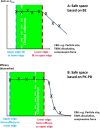
The proceedings from the 30th August 2023 (Day 2) of the workshop "Physiologically Based Biopharmaceutics Models (PBBM) Best Practices for Drug Product Quality: Regulatory and Industry Perspectives" are provided herein. Day 2 covered PBBM case studies from six regulatory authorities which provided considerations for model verification, validation, and application based on the context of use (COU) of the model. PBBM case studies to define critical material attribute (CMA) specification settings, such as active pharmaceutical ingredient (API) particle size distributions (PSDs) were shared. PBBM case studies to define critical quality attributes (CQAs) such as the dissolution specification setting or to define the bioequivalence safe space were also discussed. Examples of PBBM using the credibility assessment framework, COU and model risk assessment, as well as scientific learnings from PBBM case studies are provided. Breakout session discussions highlighted current trends and barriers to application of PBBMs including: (a) PBBM credibility assessment framework and level of validation, (b) use of disposition parameters in PBBM and points to consider when iv data are not available, (c) conducting virtual bioequivalence trials and dealing with variability, (d) model acceptance criteria, and (e) application of PBBMs for establishing safe space and failure edges.
Keywords: Bioequivalence; IVIVC; IVIVR; PBBM; bioequivalence safe space; context of use; dissolution; drug product quality; model credibility assessment framework; modeling.
Conflict of interest statement
The authors declare the following competing financial interest(s): Tycho Heimbach, David Turner, Cordula Stillhart, Philip Bransford, Xiaojun Ren, Nikunj Patel, David Sperry, Amin Rostami-Hodjegan, Viera Lukacova, Jean-Flaubert Nguefack, Tessa Carducci, Xavier Pepin, Masoud Jamei, Konstantinos Stamatopoulos, Maitri Sanghavi, Christer Tannergren, Tzuchi Rob Ju, Christian Wagner, Michael Wang, Gregory Rullo, Amitava Mitra, James Polli, Sivacharan Kollipara, Claire Mackie are employees of their respective companies and have ownership, options, and/or interests in their respective stock.
Figures


References
-
- Mackie C.; Arora S.; Seo P.; Moody R.; Rege B.; Pepin X.; Heimbach T.; Tannergren C.; Mitra A.; Suarez-Sharp S.; et al. Physiologically Based Biopharmaceutics Modeling (PBBM): Best Practices for Drug Product Quality, Regulatory and Industry Perspectives: 2023 Workshop Summary Report. Mol. Pharmaceutics 2024, 21, 2065.10.1021/acs.molpharmaceut.4c00202. - DOI - PMC - PubMed
-
- Wu F.; Shah H.; Li M.; Duan P.; Zhao P.; Suarez S.; Raines K.; Zhao Y.; Wang M.; Lin H. P.; et al. Biopharmaceutics Applications of Physiologically Based Pharmacokinetic Absorption Modeling and Simulation in Regulatory Submissions to the U.S. Food and Drug Administration for New Drugs. AAPS J. 2021, 23 (2), 31.10.1208/s12248-021-00564-2. - DOI - PubMed
-
- Physiologically Based Biopharmaceutics Modeling (PBBM) Best Practices for Drug Product Quality Workshop. University of Maryland Center of Excellence in Regulatory Science and Innovation (M-CERSI), 2023. https://cersi.umd.edu/physiologically-based-biopharmaceutics-modeling-pb... (accessed February 24th, 2024).
-
- Pepin X.; Arora S.; Borges L.; Cano-Vega M.; Carducci T.; Chatterjee P.; Chen G.; Cristofoletti R.; Dallmann A.; Delvadia P.; et al. Parameterization of Physiologically Based Biopharmaceutics Models: Workshop Summary Report. Mol. Pharmaceutics 2024, 21, 3697.10.1021/acs.molpharmaceut.4c00526. - DOI - PMC - PubMed
-
- Kuemmel C.; Yang Y.; Zhang X.; Florian J.; Zhu H.; Tegenge M.; Huang S. M.; Wang Y.; Morrison T.; Zineh I. Consideration of a Credibility Assessment Framework in Model-Informed Drug Development: Potential Application to Physiologically-Based Pharmacokinetic Modeling and Simulation. CPT Pharmacometrics Syst. Pharmacol 2020, 9 (1), 21–28. 10.1002/psp4.12479. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

