Efficacy and safety of TPO receptor agonists in treatment of ITP associated with predominantly antibody deficiencies
- PMID: 39348667
- PMCID: PMC11696776
- DOI: 10.1182/bloodadvances.2024014370
Efficacy and safety of TPO receptor agonists in treatment of ITP associated with predominantly antibody deficiencies
Abstract
Predominantly antibody deficiencies have an estimated prevalence of >1 in 25 000. Their classical phenotype entails the association of autoimmune manifestations with increased susceptibility to infections. Up to 8% of these patients ultimately develop immune thrombocytopenic purpura (ITP). Reducing the risk for infections and considering nonimmunosuppressive treatments, such as thrombopoietin receptor agonists (TPO-RAs), are important considerations for these patients. This nationwide retrospective case series assessed the outcomes and safety of TPO-RAs as treatment for ITP in adults diagnosed with predominantly antibody deficiencies. Response and complete response to treatment were defined as platelet count reaching 30 × 109/L and 100 × 109/L, respectively. We analyzed data from 28 patients. The median follow-up time after introduction of the first TPO-RAs was 33 months (range, 2 weeks to 10.6 years). After 6 weeks of follow-up, response was achieved in 24 of the 28 patients (85.7%), and among those, 21 patients (75%) displayed a complete response. At the last available follow-up visit, only 7 patients (25%) needed second-line therapies for ITP, and among those, only 5 patients (17.9%) received immunosuppressants. Only 3 patients (10.7%) reported laboratory-confirmed hepatobiliary adverse events of light or mild severity and 3 patients (10.7%) reported thrombotic events. In conclusion, TPO-RAs seemed to be an effective and safe option of treatment in these case series. Our results suggest that eltrombopag or romiplostim should be considered as second-line therapy for ITP related to predominantly antibody deficiencies.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: B.G. reports serving as an expert for Amgen, Grifols, and Novartis. H.H. reports serving as an expert for Amgen and Novartis. L.G. reports serving as an expert for Amgen, GlaxoSmithKline, Novartis, and Sanofi. M.M. reports serving as a consultant (advisory boards) for and receiving speaker fees from Grifols, Novartis, Sanofi, and Sobi. The remaining authors declare no competing financial interests.
Figures

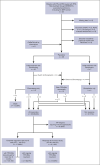
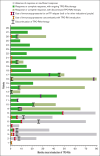
References
-
- Seidel MG, Kindle G, Gathmann B, et al. The European Society for Immunodeficiencies (ESID) registry working definitions for the clinical diagnosis of inborn errors of immunity. J Allergy Clin Immunol Pract. 2019;7(6):1763–1770. - PubMed
-
- Viallard JF, Lebail B, Begueret H, Fieschi C. Les déficits immunitaires communs variables (DICV): partie 2. Mise à jour clinique et thérapeutique. Rev Med Interne. 2021;42(7):473–481. - PubMed
-
- Boileau J, Mouillot G, Gérard L, et al. Autoimmunity in common variable immunodeficiency: correlation with lymphocyte phenotype in the French DEFI study. J Autoimmun. 2011;36(1):25–32. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

