Cellular stress and epigenetic regulation in adult stem cells
- PMID: 39348938
- PMCID: PMC11443024
- DOI: 10.26508/lsa.202302083
Cellular stress and epigenetic regulation in adult stem cells
Abstract
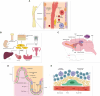
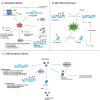
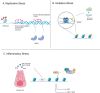
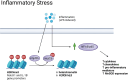
Stem cells are a unique class of cells that possess the ability to differentiate and self-renew, enabling them to repair and replenish tissues. To protect and maintain the potential of stem cells, the cells and the environment surrounding these cells (stem cell niche) are highly responsive and tightly regulated. However, various stresses can affect the stem cells and their niches. These stresses are both systemic and cellular and can arise from intrinsic or extrinsic factors which would have strong implications on overall aging and certain disease states. Therefore, understanding the breadth of drivers, namely epigenetic alterations, involved in cellular stress is important for the development of interventions aimed at maintaining healthy stem cells and tissue homeostasis. In this review, we summarize published findings of epigenetic responses to replicative, oxidative, mechanical, and inflammatory stress on various types of adult stem cells.
© 2024 Llewellyn et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
