SARS-CoV-2 spike aggravates lupus nephritis and lung fibrosis in systemic lupus erythematosus
- PMID: 39349051
- PMCID: PMC11448135
- DOI: 10.1136/lupus-2023-001104
SARS-CoV-2 spike aggravates lupus nephritis and lung fibrosis in systemic lupus erythematosus
Abstract
Objective: COVID-19 induces the development of autoimmune diseases, including SLE, which are characterised by inflammation, autoantibodies and thrombosis. However, the effects of COVID-19 on SLE remain unclear.
Methods: We investigated the effects of COVID-19 on SLE development and progression in three animal models. Plasmids encoding SARS-CoV-2 spike protein and ACE2 receptor were injected into R848-induced BALB/C lupus mice, R848-induced IL-1 receptor antagonist knockout (KO) lupus mice and MRL/lpr mice. Serum levels of albumin and autoantibodies, lymphocyte phenotypes and tissue histology were evaluated.
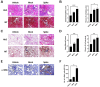
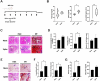
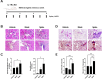
Results: In R848-induced BALB/C lupus mice, the SARS-CoV-2 spike protein increased autoantibody and albumin levels compared with vehicle and mock treatments. These mice also exhibited splenomegaly, which was further exacerbated by the spike protein. Flow cytometric analysis revealed elevated T helper 1 cell counts, and histological analysis indicated increased levels of the fibrosis marker protein α-smooth muscle actin. In KO mice, the spike protein induced splenomegaly, severe kidney damage and pronounced lung fibrosis. In the MRL/lpr group, spike protein increased the serum levels of autoantibodies, albumin and the thrombosis marker chemokine (C-X-C motif) ligand 4.
Conclusion: COVID-19 accelerated the development and progression of lupus by inducing autoantibody production, fibrosis and thrombosis.
Keywords: COVID-19; lupus erythematosus, systemic; lupus nephritis; pulmonary fibrosis.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
