Intrahepatic Exhausted Antiviral Immunity in an Immunocompetent Mouse Model of Chronic Hepatitis B
- PMID: 39349249
- PMCID: PMC11609393
- DOI: 10.1016/j.jcmgh.2024.101412
Intrahepatic Exhausted Antiviral Immunity in an Immunocompetent Mouse Model of Chronic Hepatitis B
Abstract
Background & aims: Targeting exhausted immune systems would be a promising therapeutic strategy to achieve a functional cure for HBV infection in patients with chronic hepatitis B (CHB). However, animal models recapitulating the immunokinetics of CHB are very limited. We aimed to develop an immunocompetent mouse model of CHB for intrahepatic immune profiling.
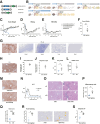
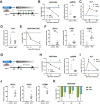
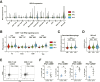
Methods: CHB mice were created by intrahepatic delivery of the Sleeping Beauty transposon vector tandemly expressing the hepatitis B virus (HBV) genome and fumarylacetoacetate hydrolase (FAH) cDNA into C57BL/6J congenic FAH knockout mice via hydrodynamic tail vein injection. We profiled the viral and intrahepatic immune kinetics in CHB mice with or without treatment with recombinant IFNα or the hepatotropic Toll-like receptor 7 agonist SA-5 using single-cell RNA-seq.
Results: CHB mice exhibited sustained HBV viremia and persistent hepatitis. They showed intrahepatic expansion of exhausted CD8+ T (Tex) cells, the frequency of which was positively associated with viral load. Recruited macrophages increased in number but impaired inflammatory responses in the liver. The cytotoxicity of mature natural killer (NK) cells also increased in CHB mice. IFNα and SA-5 treatment both resulted in viral suppression with mild hepatic flares in CHB mice. Although both treatments activated NK cells, SA-5 had the capacity to revitalize the impaired function of Tex cells and liver-recruited macrophages.
Conclusions: Our novel CHB mouse model recapitulated the intrahepatic exhausted antiviral immunity in patients with CHB, which might be able to be reinvigorated by a hepatotropic TLR7 agonist.
Keywords: Chronic Hepatitis B; HBV; Immunocompetent; Mouse Model.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- World Health Organization Fact sheet, Hepatitis B. 2022; June. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b Available at:
-
- McMahon B.J. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45–S55. - PubMed
-
- European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. - PubMed
-
- Fanning G.C., Zoulim F., Hou J.L., Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019;18:827–844. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

