Cook's balloon versus dinoprostone for Labour induction of term pregnancies with fetal GROWth restriction: study protocol for a randomised controlled trial in tertiary maternity hospitals in Spain (COLIGROW study)
- PMID: 39349375
- PMCID: PMC11448219
- DOI: 10.1136/bmjopen-2024-089628
Cook's balloon versus dinoprostone for Labour induction of term pregnancies with fetal GROWth restriction: study protocol for a randomised controlled trial in tertiary maternity hospitals in Spain (COLIGROW study)
Abstract
Introduction: Fetal growth restriction (FGR) affects about 3%-5% of term pregnancies. If prenatally detected and anterograde umbilical artery flow is preserved (stage I), it is recommended to deliver at term (≥ 37+0 weeks). In the absence of contraindications, the vaginal route is preferred, and labour induction is usually required. It has been postulated that mechanical methods for cervical ripening may have an optimal profile for the induction of term FGR fetuses since they are associated with less uterine stimulation than the standard pharmacological methods, and therefore, could be better tolerated by fetuses with reduced placental reserve. This study aims to evaluate whether cervical ripening with a Cook's balloon for the induction of labour from 37+0 weeks of gestation in the stage I FGR manages to increase the rate of vaginal delivery compared with vaginal dinoprostone.
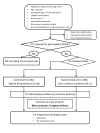
Methods and analysis: This will be an open-labelled, randomised, parallel-group clinical trial to be held in five Spanish maternities. Women aged ≥18 years with singleton pregnancies complicated with stage I FGR (defined as the presence of at least one of these two criteria: (1) estimated fetal weight (EFW) <3rd percentile; (2) EFW <10th percentile and at least one of the following: (2.1.) umbilical artery pulsatility index >95th percentile and presence of antegrade end-diastolic flow or (2.2.) Cerebroplacental ratio <5th percentile), gestational age dated by first-trimester ultrasound ≥37+0 weeks at the time of labour induction, cephalic presentation, unfavourable cervix (Bishop score <7), intact fetal membranes, no previous caesarean section and no maternal or fetal contraindications for vaginal delivery or labour induction will be 1:1 randomised by centre to labour induction with Cook's balloon (experimental arm) or dinoprostone (control arm). FGR cases with evidence of non-placental origin (major structural fetal malformations, chromosomal anomalies or congenital infection) will be excluded. The primary outcome is the achievement of a vaginal delivery and it will be assessed by comparing the rates of vaginal delivery in each group using the one-sided χ2 test at an alpha level of 0.025. The sample size has been estimated to observe an expected 84% of vaginal deliveries with Cook's balloon vs 62% with dinoprostone. Therefore, a total of 172 patients (86 per arm) are required (power of 90%, alpha level of 0.025, assuming a percentage of losses of 5%). The efficacy analysis will be performed in the intention-to-treat population. An interim analysis using a two-stage sequential design with the O'Brien-Fleming method will be applied.
Ethics and dissemination: The trial was registered in the European Union drug regulating authorities' clinical trials database (EUDRACT) (2021-001726-22) and received approval from the local Research Ethics Committee (21/728) and the Spanish Agency of Medicines and Medical Devices (AEMPS). AEMPS classified the study as a low-intervention trial. The study will be conducted in compliance with the principles of Good Clinical Practice. The study results will be disseminated through workshops and national/international conferences and published in peer-reviewed journals. In addition, they will be disclosed to patients and the public in understandable language through study newsletters and press releases to news and social media.
Protocol version: V.1.1, 18 May 2023.
Trial registration numbers: EUDRACT 2021-001726-22 and NCT05774236.
Keywords: Maternal medicine; PERINATOLOGY; Pregnant Women.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
