The critical role of Gαi3 in oral squamous cell carcinoma cell growth
- PMID: 39349425
- PMCID: PMC11443079
- DOI: 10.1038/s41420-024-02191-0
The critical role of Gαi3 in oral squamous cell carcinoma cell growth
Abstract
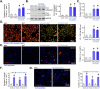
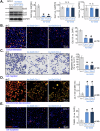
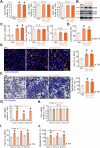
The identification of novel and effective therapeutic targets for oral squamous cell carcinoma (OSCC) is of paramount importance. This study investigates the expression, potential functions, and mechanistic insights of G protein inhibitory subunit 3 (Gαi3) in OSCC. Gαi3 is found to be upregulated in human OSCC tissues as well as in various primary and established OSCC cells. In different OSCC cells, silencing of Gαi3 through shRNA resulted in inhibited cell proliferation and migration, while also inducing apoptosis. Knockout (KO) of Gαi3 via the CRISPR/Cas9 method produced significant anti-cancer effects in OSCC cells. Conversely, ectopic overexpression of Gαi3 enhanced OSCC cell growth, promoting cell proliferation and migration. Gαi3 plays a crucial role in activating the Akt-mTOR signaling pathway in OSCC cells. Silencing or KO of Gαi3 led to decreased phosphorylation levels of Akt and S6K, whereas overexpression of Gαi3 increased their phosphorylation. Restoration of Akt-mTOR activation through a constitutively active mutant Akt1 mitigated the anti-OSCC effects induced by Gαi3 shRNA. In vivo, Gαi3 silencing significantly suppressed the growth of subcutaneous OSCC xenografts in nude mice, concomitant with inactivation of the Akt-mTOR pathway and induction of apoptosis. Collectively, these findings underscore the critical role of Gαi3 in OSCC cell growth both in vitro and in vivo.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Chamoli A, Gosavi AS, Shirwadkar UP, Wangdale KV, Behera SK, Kurrey NK, et al. Overview of oral cavity squamous cell carcinoma: risk factors, mechanisms, and diagnostics. Oral Oncol. 2021;121:105451. - PubMed
-
- Massano J, Regateiro FS, Januario G, Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:67–76. - PubMed
-
- Omura K. Current status of oral cancer treatment strategies: surgical treatments for oral squamous cell carcinoma. Int J Clin Oncol. 2014;19:423–30. - PubMed
-
- Bhatia A, Burtness B. Treating head and neck cancer in the age of immunotherapy: a 2023 update. Drugs. 2023;83:217–48. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

