Fatty acid synthase (FASN) is a tumor-cell-intrinsic metabolic checkpoint restricting T-cell immunity
- PMID: 39349429
- PMCID: PMC11442875
- DOI: 10.1038/s41420-024-02184-z
Fatty acid synthase (FASN) is a tumor-cell-intrinsic metabolic checkpoint restricting T-cell immunity
Abstract
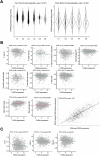
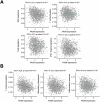
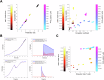
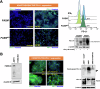
Fatty acid synthase (FASN)-catalyzed endogenous lipogenesis is a hallmark of cancer metabolism. However, whether FASN is an intrinsic mechanism of tumor cell defense against T cell immunity remains unexplored. To test this hypothesis, here we combined bioinformatic analysis of the FASN-related immune cell landscape, real-time assessment of cell-based immunotherapy efficacy in CRISPR/Cas9-based FASN gene knockout (FASN KO) cell models, and mathematical and mechanistic evaluation of FASN-driven immunoresistance. FASN expression negatively correlates with infiltrating immune cells associated with cancer suppression, cytolytic activity signatures, and HLA-I expression. Cancer cells engineered to carry a loss-of-function mutation in FASN exhibit an enhanced cytolytic response and an accelerated extinction kinetics upon interaction with cytokine-activated T cells. Depletion of FASN results in reduced carrying capacity, accompanied by the suppression of mitochondrial OXPHOS and strong downregulation of electron transport chain complexes. Targeted FASN depletion primes cancer cells for mitochondrial apoptosis as it synergizes with BCL-2/BCL-XL-targeting BH3 mimetics to render cancer cells more susceptible to T-cell-mediated killing. FASN depletion prevents adaptive induction of PD-L1 in response to interferon-gamma and reduces constitutive overexpression of PD-L1 by abolishing PD-L1 post-translational palmitoylation. FASN is a novel tumor cell-intrinsic metabolic checkpoint that restricts T cell immunity and may be exploited to improve the efficacy of T cell-based immunotherapy.
© 2024. The Author(s).
Conflict of interest statement
Joaquim Bosch-Barrera reports grant funding and personal fees from Pfizer, MSD Spain, BMS, AstraZeneca, Novartis, Boehringer-Ingelheim, Vifor, Sanofi, and LEO Pharma outside the submitted work. These companies had no role in the design of the study, in the collection, analysis, or interpretation of the data; in writing the manuscript; or in the decision to submit the results for publication. All of the other authors declare that they have no known competing financial interests or personal relationships that could potentially influence the work reported in this article.
Figures



References
Grants and funding
- BC151072P1/U.S. Department of Defense (United States Department of Defense)
- CP20/00003/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- PI21/0136/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- CM22/00276/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- BC151072/U.S. Department of Defense (United States Department of Defense)
- R01 CA116623/CA/NCI NIH HHS/United States
- SLT017/20/000072/Generalitat de Catalunya (Government of Catalonia)
- SGR 2021-00184/Generalitat de Catalunya (Government of Catalonia)
- PI22/00297/Ministry of Economy and Competitiveness | Instituto de Salud Carlos III (Institute of Health Carlos III)
- 2022 INV-1 00001/Generalitat de Catalunya (Government of Catalonia)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

