Identification of Gαi3 as a promising molecular oncotarget of pancreatic cancer
- PMID: 39349432
- PMCID: PMC11442978
- DOI: 10.1038/s41419-024-07079-6
Identification of Gαi3 as a promising molecular oncotarget of pancreatic cancer
Abstract
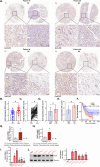
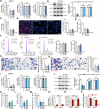
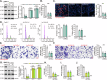
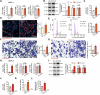
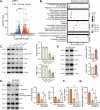
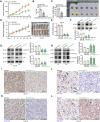
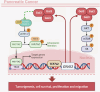
The increasing mortality rate of pancreatic cancer globally necessitates the urgent identification for novel therapeutic targets. This study investigated the expression, functions, and mechanistic insight of G protein inhibitory subunit 3 (Gαi3) in pancreatic cancer. Bioinformatics analyses reveal that Gαi3 is overexpressed in human pancreatic cancer, correlating with poor prognosis, higher tumor grade, and advanced classification. Elevated Gαi3 levels are also confirmed in human pancreatic cancer tissues and primary/immortalized cancer cells. Gαi3 shRNA or knockout (KO) significantly reduced cell viability, proliferation, cell cycle progression, and mobility in primary/immortalized pancreatic cancer cells. Conversely, Gαi3 overexpression enhanced pancreatic cancer cell growth. RNA-sequencing and bioinformatics analyses of Gαi3-depleted cells indicated Gαi3's role in modulating the Akt-mTOR and PKA-Hippo-YAP pathways. Akt-S6 phosphorylation was decreased in Gαi3-depleted cells, but was increased with Gαi3 overexpression. Additionally, Gαi3 depletion elevated PKA activity and activated the Hippo pathway kinase LATS1/2, leading to YAP/TAZ inactivation, while Gαi3 overexpression exerted the opposite effects. There is an increased binding between Gαi3 promoter and the transcription factor TCF7L2 in pancreatic cancer tissues and cells. Gαi3 expression was significantly decreased following TCF7L2 silencing, but increased with TCF7L2 overexpression. In vivo, intratumoral injection of Gαi3 shRNA-expressing adeno-associated virus significantly inhibited subcutaneous pancreatic cancer xenografts growth in nude mice. A significant growth reduction was also observed in xenografts from Gαi3 knockout pancreatic cancer cells. Akt-mTOR inactivation and increased PKA activity coupled with YAP/TAZ inactivation were also detected in xenograft tumors upon Gαi3 depletion. Furthermore, bioinformatic analysis and multiplex immunohistochemistry (mIHC) staining on pancreatic cancer tissue microarrays showed a reduced proportion of M1-type macrophages and an increase in PD-L1 positive cells in Gαi3-high pancreatic cancer tissues. Collectively, these findings highlight Gαi3's critical role in promoting pancreatic cancer cell growth, potentially through the modulation of the Akt-mTOR and PKA-Hippo-YAP pathways and its influence on the immune landscape.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: a cancer J clinicians. 2020;70:7–30. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

