CRISPR/Cas9 editing of NKG2A improves the efficacy of primary CD33-directed chimeric antigen receptor natural killer cells
- PMID: 39349459
- PMCID: PMC11442982
- DOI: 10.1038/s41467-024-52388-1
CRISPR/Cas9 editing of NKG2A improves the efficacy of primary CD33-directed chimeric antigen receptor natural killer cells
Abstract
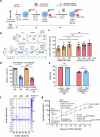
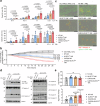
Chimeric antigen receptor (CAR)-modified natural killer (NK) cells show antileukemic activity against acute myeloid leukemia (AML) in vivo. However, NK cell-mediated tumor killing is often impaired by the interaction between human leukocyte antigen (HLA)-E and the inhibitory receptor, NKG2A. Here, we describe a strategy that overcomes CAR-NK cell inhibition mediated by the HLA-E-NKG2A immune checkpoint. We generate CD33-specific, AML-targeted CAR-NK cells (CAR33) combined with CRISPR/Cas9-based gene disruption of the NKG2A-encoding KLRC1 gene. Using single-cell multi-omics analyses, we identified transcriptional features of activation and maturation in CAR33-KLRC1ko-NK cells, which are preserved following exposure to AML cells. Moreover, CAR33-KLRC1ko-NK cells demonstrate potent antileukemic killing activity against AML cell lines and primary blasts in vitro and in vivo. We thus conclude that NKG2A-deficient CAR-NK cells have the potential to bypass immune suppression in AML.
© 2024. The Author(s).
Conflict of interest statement
E.U. has a sponsored research project with Gilead and BMS and acts as medical advisor of Phialogics and CRIION. T.O. has disclosures to Merck KGaA: Honoraria; Gilead: Research Funding; Merck KGaA: Research Funding; Roche: Honoraria. O.P. has received honoraria or travel support from Astellas, Gilead, Jazz, MSD, Neovii Biotech, Novartis, Pfizer, and Therakos, he has received research support from Gilead, Incyte, Jazz, Neovii Biotech, and Takeda and is member of advisory boards to Jazz, Gilead, MSD, Omeros, Priothera, Shionogi, and SOBI. N.M. is employee of Miltenyi Biotec. T.B., P.W., E.U. have filed patents on data partly published in this manuscript: PCT/EP2024/060767: Treatment of leukemia with engineered immune checkpoint inactivated CAR-NK cells and CAR-T cells (U31175WO). No competing interests exists for the remaining authors.
Figures





References
-
- Munshi, N. C. et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med.384, 705–716 (2021). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

