Pharmacokinetics and pharmacogenomics of ribociclib in black patients with metastatic breast cancer the LEANORA study
- PMID: 39349477
- PMCID: PMC11442496
- DOI: 10.1038/s41523-024-00692-w
Pharmacokinetics and pharmacogenomics of ribociclib in black patients with metastatic breast cancer the LEANORA study
Abstract
Underrepresented populations' participation in clinical trials remains limited, and the potential impact of genomic variants on drug metabolism remains elusive. This study aimed to assess the pharmacokinetics (PK) and pharmacogenomics (PGx) of ribociclib in self-identified Black women with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2) advanced breast cancer. LEANORA (NCT04657679) was a prospective, observational, multicenter cohort study involving 14 Black women. PK and PGx were evaluated using tandem mass spectrometry and PharmacoScan™ microarray (including CYP3A5*3, *6, and *7). CYP3A5 phenotypes varied among participants: 7 poor metabolizers (PM), 6 intermediate metabolizers (IM), and one normal metabolizer (NM). The area under the curve did not significantly differ between PMs (39,230 h*ng/mL) and IM/NMs (43,546 h*ng/mL; p = 0.38). The incidence of adverse events (AEs) was also similar. We found no association between CYP3A5 genotype and ribociclib exposure. Continued efforts are needed to include diverse populations in clinical trials to ensure equitable treatment outcomes.
© 2024. The Author(s).
Conflict of interest statement
D.M.S. reports research funding to institution from Kailos Genetics, Inc. MTT reports receiving honoria from AstraZeneca, Incyce, Otsuka, Sanofi Pasteur, consulting for American Gene Technologies, and receiving research funding to institution from Genentech. N.H.B. reports they or an immediate family member have consulted for Catena, a leadership role for Seagen, stock or ownership in Seagen, Lilly, Gilead Sciences, and Pfizer. C.G. reports consulting for Daiichi Sankyo, Illy, Biotheranostics, and Pfizer and Speakers’ Bureau for Daiichi Sankyo/UCB Japan. K.D.W. reports consults for Biotheranostics and receiving honoraria from MHJ Life Sciences. C.B.M. reports receiving research funding to institution from Pfizer and Cantex Pharmaceuticals. C.I. reports consulting for Pfizer, Novartis, Puma Biotechnology, Seagen, Ion Solutions, AstraZeneca/MedImmune, Gilead Sciences, receiving travel support from Pfizer, holding patents, royalties, or other intellectual property from McGraw Hill Publishing, UpToDate (Wolters Kluwer), Elsevier, receiving honoraria from Pfizer, receiving research funding to institution from Tesaro, Merck, Seagen, Pfizer, GlaxoSmithKline, AstraZeneca, Novartis, Genentech/Rosche, Bristol-Myers Squibb/Celgene, and other relationships with Side-Out Foundation, M.J.H./P.E.R., Curio/Vaniam Group, and Medscape. W.D.F. reports research funding to institution from Celgene, Astellas Pharma, Nerviano Medical Sciences, Pfizer, NovaRX, TRACON Pharma, Biocompatibles, and Propella Therapeutics. SM Swain reports consulting for Genetech/Roche, Daiichi Sankyo, Molecular Templates, AstraZeneca, Aventis Pharma, Jaguar Health, a leadership role at Seagen, receiving travel support from Daiichi Sankyo, Aventis Pharma, Genentech/Roche, and Chugai/Roche, stock or ownership in Seagen, receiving honoraria from Chugai/Roche, and other relationships with Roche, AstraZeneca, and Genentech/Roche. I.S., C.P., T.S., K.T.S., A.C., S.S., H.C.W., N.A., S.R.T., N.S., G.C.N., S.K.M.: Nothing to declare.
Figures
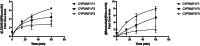

Update of
-
Pharmacokinetics and Pharmacogenomics of Ribociclib in Black Patients with Metastatic Breast Cancer: The LEANORA study.Res Sq [Preprint]. 2024 Aug 13:rs.3.rs-4656461. doi: 10.21203/rs.3.rs-4656461/v1. Res Sq. 2024. Update in: NPJ Breast Cancer. 2024 Sep 30;10(1):84. doi: 10.1038/s41523-024-00692-w. PMID: 39184092 Free PMC article. Updated. Preprint.
References
-
- Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA: A Cancer J. Clin.74, 12–49 (2024). - PubMed
-
- Finn, R. S. et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): Analyses from PALOMA-2. J. Clin. Oncol.40, LBA1003–LBA1003 (2022). - DOI
-
- Cristofanilli, M. et al. Overall survival (OS) with palbociclib (PAL) + fulvestrant (FUL) in women with hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) advanced breast cancer (ABC): Updated analyses from PALOMA-3. J. Clin. Oncol.39, 1000–1000 (2021). - DOI
Grants and funding
- ASCO Young Investigator Award (PI)/Conquer Cancer Foundation (Conquer Cancer Foundation of the American Society of Clinical Oncology)
- UL1 RR031975/RR/NCRR NIH HHS/United States
- UL1 TR000101/TR/NCATS NIH HHS/United States
- P30 CA051008/CA/NCI NIH HHS/United States
- Core grant (P30CA051008), Grant # UL1TR000101 (previously UL1RR031975)/U.S. Department of Health & Human Services | NIH | National Center for Advancing Translational Sciences (NCATS)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

