New Bacillus paralicheniformis strain with high proteolytic and keratinolytic activity
- PMID: 39349615
- PMCID: PMC11444040
- DOI: 10.1038/s41598-024-73468-8
New Bacillus paralicheniformis strain with high proteolytic and keratinolytic activity
Abstract
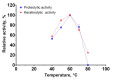
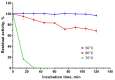
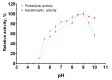
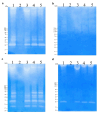
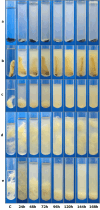
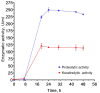
Bacillus paralicheniformis T7, which exhibits high proteolytic and keratinolytic activities, was isolated from soil in Kazakhstan. Its secreted proteases were thermostable and alkaline, demonstrating maximum activity at 70 °C and pH 9.0. The proteases and keratinases of this strain were sensitive to Ni2+, Co2+, Mn2+, and Cd2+, with Cu2+, Co2+ and Cd2+ negatively affecting keratinolytic activity, and Fe3+ ions have a strong inhibitory effect on proteolytic and keratinolytic activity. Seven proteases were identified in the enzymatic extract of B. paralicheniformis T7: four from the serine peptidase family and three from the metallopeptidase family. The proteases hydrolyzed 1 mg of casein, hemoglobin, gelatin, ovalbumin, bovine serum albumin, or keratin within 15 s to 30 min. The high keratinolytic activity of this strain was confirmed through the degradation of chicken feathers, horns, hooves, wool, and cattle hide. Chicken feathers were hydrolyzed in 4 days, and the degrees of hydrolysis for cattle hide, wool, hoof, and horn after 7 days of cultivation were 97.2, 34.5, 29.6, and 3.6%, respectively. During submerged fermentation with feather medium in a laboratory bioreactor, the strain secreted enzymes with 249.20 ± 7.88 U/mL protease activity after 24 h. Thus, B. paralicheniformis T7 can be used to produce proteolytic and keratinolytic enzymes for application in processing proteinaceous raw materials and keratinous animal waste.
Keywords: Bacillus paralicheniformis T7; Keratinases; Proteases.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
High keratinase and other types of hydrolase activity of the new strain of Bacillus paralicheniformis.PLoS One. 2024 Oct 25;19(10):e0312679. doi: 10.1371/journal.pone.0312679. eCollection 2024. PLoS One. 2024. PMID: 39453952 Free PMC article.
-
Keratinolytic enzyme-mediated biodegradation of recalcitrant poultry feathers waste by newly isolated Bacillus sp. NKSP-7 under submerged fermentation.Folia Microbiol (Praha). 2020 Oct;65(5):823-834. doi: 10.1007/s12223-020-00793-6. Epub 2020 May 16. Folia Microbiol (Praha). 2020. PMID: 32415568
-
Chicken feathers: a complex substrate for the co-production of alpha-amylase and proteases by B. licheniformis NH1.J Ind Microbiol Biotechnol. 2010 Sep;37(9):983-90. doi: 10.1007/s10295-010-0792-8. Epub 2010 Aug 8. J Ind Microbiol Biotechnol. 2010. PMID: 20694741
-
Feather degradation by keratinolytic bacteria and biofertilizing potential for sustainable agricultural production.J Basic Microbiol. 2019 Jan;59(1):4-13. doi: 10.1002/jobm.201800434. Epub 2018 Oct 24. J Basic Microbiol. 2019. PMID: 30353928 Review.
-
Industrial sustainability of microbial keratinases: production and potential applications.World J Microbiol Biotechnol. 2021 Apr 17;37(5):86. doi: 10.1007/s11274-021-03052-z. World J Microbiol Biotechnol. 2021. PMID: 33864165 Review.
Cited by
-
Bacillus paralicheniformis LN33 fermented feed improves growth performance in Cherry Valley ducks by enhancing immune function and intestinal barrier integrity.Front Vet Sci. 2025 Jul 23;12:1619287. doi: 10.3389/fvets.2025.1619287. eCollection 2025. Front Vet Sci. 2025. PMID: 40771965 Free PMC article.
References
-
- Li, X. et al. Novel detection method for evaluating the activity of an alkaline serine protease from Bacillus clausii. J. Agric. Food Chem.70, 3765–3774. 10.1021/acs.jafc.2c00358 (2022). - PubMed
-
- Rehman, R. et al. Catalytic role of thermostable metalloproteases from Bacillus subtilis KT004404 as dehairing and destaining agent. Appl. Biochem. Biotechnol.181, 434–450. 10.1007/s12010-016-2222-5 (2017). - PubMed
-
- Kirk, O., Borchert, T. V. & Fuglsang, C. C. Industrial enzyme applications. Curr. Opin. Biotechnol.13, 345–351. 10.1016/s0958-1669(02)00328-2 (2002). - PubMed
-
- Yu, P., Wang, X., Huang, X., Ren, Q. & Yan, T. Purification and characterization of a propanol-tolerant neutral protease from Bacillus sp. ZG20. Prep. Biochem. Biotechnol.49, 718–726. 10.1080/10826068.2019.1605526 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

