Leishmania major-induced alteration of host cellular and systemic copper homeostasis drives the fate of infection
- PMID: 39349621
- PMCID: PMC11442737
- DOI: 10.1038/s42003-024-06716-2
Leishmania major-induced alteration of host cellular and systemic copper homeostasis drives the fate of infection
Abstract
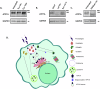
Copper plays a key role in host-pathogen interaction. We find that during Leishmania major infection, the parasite-harboring macrophage regulates its copper homeostasis pathway in a way to facilitate copper-mediated neutralization of the pathogen. Copper-ATPase ATP7A transports copper to amastigote-harboring phagolysosomes to induce stress on parasites. Leishmania in order to evade the copper stress, utilizes a variety of manipulative measures to lower the host-induced copper stress. It induces deglycosylation and degradation of host-ATP7A and downregulation of copper importer, CTR1 by cysteine oxidation. Additionally, Leishmania induces CTR1 endocytosis that arrests copper uptake. In mouse model of infection, we report an increase in systemic bioavailable copper in infected animals. Heart acts as the major organ for diverting its copper reserves to systemic circulation to fight-off infection by downregulating its CTR1. Our study explores reciprocal mechanism of manipulation of host copper homeostasis pathway by macrophage and Leishmania to gain respective advantages in host-pathogen interaction.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
A novel leishmanial copper P-type ATPase plays a vital role in parasite infection and intracellular survival.J Biol Chem. 2022 Feb;298(2):101539. doi: 10.1016/j.jbc.2021.101539. Epub 2021 Dec 25. J Biol Chem. 2022. PMID: 34958799 Free PMC article.
-
Intracellular Survival of Leishmania major Depends on Uptake and Degradation of Extracellular Matrix Glycosaminoglycans by Macrophages.PLoS Pathog. 2015 Sep 3;11(9):e1005136. doi: 10.1371/journal.ppat.1005136. eCollection 2015 Sep. PLoS Pathog. 2015. PMID: 26334531 Free PMC article.
-
SP1/CTR1-mediated oxidative stress-induced cuproptosis in intervertebral disc degeneration.Biofactors. 2024 Sep-Oct;50(5):1009-1023. doi: 10.1002/biof.2052. Epub 2024 Apr 10. Biofactors. 2024. PMID: 38599595
-
Cutaneous leishmaniasis: Distinct functions of dendritic cells and macrophages in the interaction of the host immune system with Leishmania major.Int J Med Microbiol. 2018 Jan;308(1):206-214. doi: 10.1016/j.ijmm.2017.11.002. Epub 2017 Nov 6. Int J Med Microbiol. 2018. PMID: 29129568 Review.
-
Interaction of Leishmania with the host macrophage.Trends Parasitol. 2002 Aug;18(8):332-4. doi: 10.1016/s1471-4922(02)02352-8. Trends Parasitol. 2002. PMID: 12377273 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

