Low-dose spironolactone and cardiovascular outcomes in moderate stage chronic kidney disease: a randomized controlled trial
- PMID: 39349629
- PMCID: PMC11753262
- DOI: 10.1038/s41591-024-03263-5
Low-dose spironolactone and cardiovascular outcomes in moderate stage chronic kidney disease: a randomized controlled trial
Abstract
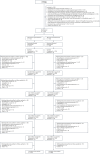
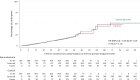
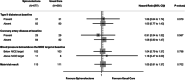
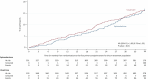
Chronic kidney disease (CKD) is associated with a substantial risk of progression to end-stage renal disease and vascular events. The nonsteroidal mineralocorticoid receptor antagonist (MRA), finerenone, offers cardiorenal protection for people with CKD and diabetes, but there is uncertainty if the steroidal MRA, spironolactone, provides the same protection. In this prospective, randomized, open, blinded endpoint trial, we assessed the effectiveness of 25 mg spironolactone in addition to usual care or usual care alone for reducing cardiovascular outcomes in stage 3b CKD among an older community cohort (mean age = 74.8 years and s.d. = 8.1). We recruited 1,434 adults from English primary care, of whom 1,372 (96%) were included in the primary analysis. The primary outcome was time from randomization until the first occurrence of death, hospitalization for heart disease, stroke, heart failure, transient ischemic attack or peripheral arterial disease, or first onset of any condition listed not present at baseline. Across 3 years of follow-up, the primary endpoint occurred in 113 of 677 participants randomized to spironolactone (16.7%) and 111 of 695 participants randomized to usual care (16.0%) with no significant difference between groups (hazard ratio = 1.05, 95% confidence interval: 0.81-1.37). Two-thirds of participants randomized to spironolactone stopped treatment within 6 months, predominantly because they met prespecified safety stop criteria. The most common reason for stopping spironolactone was a decrease in the estimated glomerular filtration rate that met prespecified stop criteria (n = 239, 35.4%), followed by participants being withdrawn due to treatment side effects (n = 128, 18.9%) and hyperkalemia (n = 54, 8.0%). In conclusion, we found that spironolactone was frequently discontinued due to safety concerns, with no evidence that it reduced cardiovascular outcomes in people with stage 3b CKD. Spironolactone should not be used for people with stage 3b CKD without another explicit treatment indication. ClinicalTrials.gov registration: ISRCTN44522369 .
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

