Therapeutic targeting of senescent cells in the CNS
- PMID: 39349637
- PMCID: PMC11927922
- DOI: 10.1038/s41573-024-01033-z
Therapeutic targeting of senescent cells in the CNS
Abstract
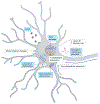
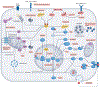
Senescent cells accumulate throughout the body with advanced age, diseases and chronic conditions. They negatively impact health and function of multiple systems, including the central nervous system (CNS). Therapies that target senescent cells, broadly referred to as senotherapeutics, recently emerged as potentially important treatment strategies for the CNS. Promising therapeutic approaches involve clearing senescent cells by disarming their pro-survival pathways with 'senolytics'; or dampening their toxic senescence-associated secretory phenotype (SASP) using 'senomorphics'. Following the pioneering discovery of first-generation senolytics dasatinib and quercetin, dozens of additional therapies have been identified, and several promising targets are under investigation. Although potentially transformative, senotherapies are still in early stages and require thorough testing to ensure reliable target engagement, specificity, safety and efficacy. The limited brain penetrance and potential toxic side effects of CNS-acting senotherapeutics pose challenges for drug development and translation to the clinic. This Review assesses the potential impact of senotherapeutics for neurological conditions by summarizing preclinical evidence, innovative methods for target and biomarker identification, academic and industry drug development pipelines and progress in clinical trials.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests
M.X. is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. A.A.J. is an employee of Merck Sharp & Dohme (UK) Limited. M.E.O. has a patent pending, ‘Detecting and treating conditions associated with neuronal senescence’.
Figures

References
-
- Hayflick L & Moorhead PS The serial cultivation of human diploid cell strains. Exp. Cell Res 25, 585–621 (1961). - PubMed
-
- Lundblad V & Szostak JW A mutant with a defect in telomere elongation leads to senescence in yeast. Cell 57, 633–643 (1989). - PubMed
-
- Harley CB, Futcher AB & Greider CW Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460 (1990). - PubMed
-
- Herbig U, Ferreira M, Condel L, Carey D & Sedivy JM Cellular senescence in aging primates. Science 311, 1257 (2006). - PubMed
-
- Minamino T et al. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105, 1541–1544 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

