Structural phase transition in NH₄F under extreme pressure conditions
- PMID: 39349697
- PMCID: PMC11443071
- DOI: 10.1038/s42004-024-01309-w
Structural phase transition in NH₄F under extreme pressure conditions
Erratum in
-
Author Correction: Structural phase transition in NH4F under extreme pressure conditions.Commun Chem. 2025 Feb 24;8(1):57. doi: 10.1038/s42004-025-01457-7. Commun Chem. 2025. PMID: 39994378 Free PMC article. No abstract available.
Abstract
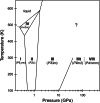
Ammonium fluoride (NH₄F) exhibits a variety of crystalline phases depending on temperature and pressure. By employing Raman spectroscopy and synchrotron X-ray diffraction beyond megabar pressures (up to 140 GPa), we have here observed a novel dense solid phase of NH₄F, characterised by the tetragonal P4/nmm structure also observed in other ammonium halides under less extreme pressure conditions, typically a few GPa. Using detailed ab-initio calculations and reevaluating earlier theoretical models pertaining to other ammonium halides, we examine the microscopic mechanisms underlying the transition from the low-pressure cubic phase (P-43m) to the newly identified high-pressure tetragonal phase (P4/nmm). Notably, NH₄F exhibits distinctive properties compared to its counterparts, resulting in a significantly broader pressure range over which this transition unfolds, facilitating the identification of its various stages. Our analysis points to a synergistic interplay driving the transition to the P4/nmm phase, which we name phase VIII. At intermediate pressures (around 40 GPa), a displacive transition of fluorine ions initiates a tetragonal distortion of the cubic phase. Subsequently, at higher pressures (around 115 GPa), every second ammonium ion undergoes a rotational shift, adopting an anti-tetrahedral arrangement. This coupled effect orchestrates the transition process, leading to the formation of the tetragonal phase.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Bellin, C. et al. Disorder-order phase transition at high pressure in ammonium fluoride. Phys. Rev. B96, 094110 (2017). - DOI
-
- Heyns, A. M. The effect of pressure on the Raman spectrum of NH4Cl. J. Phys. Chem. Solids41, 769–776 (1980). - DOI
-
- Schwake, A., Hirsch, K. R. & Holzapfel, W. B. Raman spectra of NH4Br at high pressure and the location of the IV–V phase transition. J. Chem. Phys.75, 2532–2534 (1981). - DOI
-
- Heyns, A. M., Hirsch, K. R. & Holzapfel, W. B. The effect of pressure on the Raman spectrum of NH4I. J. Chem. Phys.73, 105–119 (1980). - DOI
LinkOut - more resources
Full Text Sources

