Efficacy of epidermal growth factor in suppressing inflammation and proliferation in pterygial fibroblasts through interactions with microenvironmental M1 macrophages
- PMID: 39349715
- PMCID: PMC11442942
- DOI: 10.1038/s41598-024-74413-5
Efficacy of epidermal growth factor in suppressing inflammation and proliferation in pterygial fibroblasts through interactions with microenvironmental M1 macrophages
Abstract
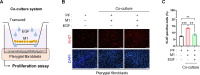
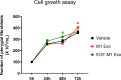
The protein epidermal growth factor (EGF), which plays a crucial role in promoting cell proliferation and survival, has recently demonstrated potential in reducing inflammation. In this study, we examined the impact of EGF on the anti-inflammatory and anti-proliferative properties of pterygium, a prevalent hypervascular proliferative disease affecting the ocular surface. In surgically excised tissues, markers for fibrotic and inflammatory signals, including VIM, ACTA2, FAP, MMP2, VCAM1, ICAM1, CD86, IL6, and IL1B were upregulated in the pterygium body stroma compared to the normal conjunctival stroma. EGF exerted anti-inflammatory and anti-vasculogenic effects on pterygial fibroblasts when co-cultured with M1 macrophages. Moreover, exosomes derived from EGF-preconditioned M1 macrophages suppressed the heightened inflammatory and vasculogenic signals in pterygial fibroblasts induced by exosomes from M1 macrophages. Paradoxically, the proliferation of pterygial fibroblasts was inhibited by EGF in the in vitro microenvironment with M1 macrophages, despite EGF being known as a growth factor. EGF-preconditioning of M1 macrophages rescued the increased proliferation of pterygial fibroblasts induced by exosomes from M1 macrophages. In conclusion, our findings demonstrate that EGF effectively mitigates inflammation and proliferation in pterygial fibroblasts within a microenvironment containing M1 macrophages.
Keywords: EGF; Exosome; Inflammation; Proliferation; Pterygial fibroblast; Pterygium.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
M2a Macrophage-Dominant Microenvironment in Inflammation Attenuation and Wound Healing of Human Corneal Endothelial Cells.Invest Ophthalmol Vis Sci. 2025 Jun 2;66(6):35. doi: 10.1167/iovs.66.6.35. Invest Ophthalmol Vis Sci. 2025. PMID: 40492988 Free PMC article.
-
Upregulation of Transient Receptor Potential Vanilloid Type-1 Channel Activity and Ca2+ Influx Dysfunction in Human Pterygial Cells.Invest Ophthalmol Vis Sci. 2016 May 1;57(6):2564-77. doi: 10.1167/iovs.16-19170. Invest Ophthalmol Vis Sci. 2016. PMID: 27163769
-
Downregulation of c-Myc in pterygium and cultured pterygial cells.Clin Exp Ophthalmol. 2011 Nov;39(8):784-92. doi: 10.1111/j.1442-9071.2011.02531.x. Epub 2011 Apr 27. Clin Exp Ophthalmol. 2011. PMID: 22050566
-
Targeting platelet-derived growth factor receptor β inhibits the proliferation and motility of human pterygial fibroblasts.Expert Opin Ther Targets. 2019 Sep;23(9):805-817. doi: 10.1080/14728222.2019.1653281. Epub 2019 Aug 12. Expert Opin Ther Targets. 2019. PMID: 31385548
-
Pterygia: pathogenesis and the role of subconjunctival bevacizumab in treatment.Semin Ophthalmol. 2009 May-Jun;24(3):130-4. doi: 10.1080/08820530902801106. Semin Ophthalmol. 2009. PMID: 19437347 Review.
Cited by
-
Increasing the concentration of plasma molecules improves the biological activity of platelet-rich plasma for tissue regeneration.Sci Rep. 2025 Feb 6;15(1):4523. doi: 10.1038/s41598-025-88918-0. Sci Rep. 2025. PMID: 39915642 Free PMC article.
-
N-acetyl L-cysteine and Growth Factors Impede Endoplasmic Reticulum Stress and Inflammatory Responses in Astrocytes to Amyloid-β in Serum-free Culture.Ann Neurosci. 2025 Jul 7:09727531251340150. doi: 10.1177/09727531251340150. Online ahead of print. Ann Neurosci. 2025. PMID: 40636588 Free PMC article.
-
M2a Macrophage-Dominant Microenvironment in Inflammation Attenuation and Wound Healing of Human Corneal Endothelial Cells.Invest Ophthalmol Vis Sci. 2025 Jun 2;66(6):35. doi: 10.1167/iovs.66.6.35. Invest Ophthalmol Vis Sci. 2025. PMID: 40492988 Free PMC article.
-
Efferocytosis by Macrophages Attenuates Inflammatory Responses Following Ultraviolet B-Induced Apoptosis in Corneal Stromal Cells.Invest Ophthalmol Vis Sci. 2025 Jul 1;66(9):49. doi: 10.1167/iovs.66.9.49. Invest Ophthalmol Vis Sci. 2025. PMID: 40673741 Free PMC article.
References
-
- Kim, K. W., Park, S. H. & Kim, J. C. Fibroblast biology in pterygia. Exp. Eye Res. 142, 32–39 (2016). - PubMed
-
- Di Girolamo, N. Signalling pathways activated by ultraviolet radiation: role in ocular and cutaneous health. Curr. Pharm. Des. 16, 1358–1375 (2010). - PubMed
-
- Di Girolamo, N., Kumar, R. K., Coroneo, M. T. & Wakefield, D. UVB-mediated induction of interleukin-6 and – 8 in pterygia and cultured human pterygium epithelial cells. Invest. Ophthalmol. Vis. Sci. 43, 3430–3437 (2002). - PubMed
-
- Kennedy, M. et al. Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Invest. Ophthalmol. Vis. Sci. 38, 2483–2491 (1997). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

