Activity of the hypothalamic neuropeptide Y increases in adult and decreases in old rats
- PMID: 39349740
- PMCID: PMC11442438
- DOI: 10.1038/s41598-024-73825-7
Activity of the hypothalamic neuropeptide Y increases in adult and decreases in old rats
Abstract
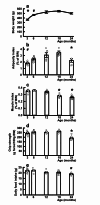
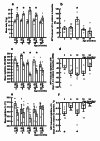
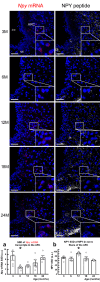
Middle-aged obesity and aging anorexia with muscle loss (sarcopenia) of old people present public health burden. These alterations may appear both in humans and rodents suggesting the role for regulatory alterations. Previously, we demonstrated that biphasic changes in the weight-reducing (catabolic) effects of neuropeptides of the hypothalamus-adipose tissue axis (e.g. leptin) may contribute to both trends. With regard to the anabolic effects of the hypothalamic neuropeptide Y (NPY) inhibited by leptin, we hypothesized non-linear age-related changes with shifts in the opposite directions. We investigated the orexigenic and hypometabolic effects of intracerebroventricularly administered NPY (hyperphagia induced by NPY injection or changes in food intake, body weight, heart rate, body temperature, locomotor activity during a 7-day NPY infusion), the immunoreactivity and gene expression of NPY in the hypothalamic arcuate nucleus of male Wistar rats of five age groups from young to old. The orexigenic/hypometabolic efficacy and the immunoreactivity of NPY increased in middle-aged animals preceding the peak of adiposity observed in aging rats, then decreased preceding anorexia and weight loss in old rats. These shifts may contribute to the development of both age-related obesity and aging anorexia, sarcopenia, and should be considered in future drug development targeting the NPY system.
Keywords: Aging anorexia; Metabolism; Obesity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- FK124188/National Research, Development and Innovation Fund of Hungary
- K138452/National Research, Development and Innovation Fund of Hungary
- TKP2021-EGA-16/Thematic Excellence Program 2021 Health Sub-program of the Ministry for Innovation and Technology in Hungary, within the framework of the EGA-16 project of Pécs University
- HUN-REN-TKI14016/Hungarian Research Network
- ÁOK_KA_2022_29/Medical School of the University of Pecs, Hungary
- ÁOK_KA_2021_37/Medical School of the University of Pecs, Hungary
- ÁOK_KA_2019_44/Medical School of the University of Pecs, Hungary
- BO/00750/22/5/János Bolyai Research Scholarship of the Hungarian Academy of Sciences
- ÚNKP-23-5-PTE-1991/New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund
- RRF, RRF-2.3.1-21-2022-00011/European Union's Recovery and Resilience Facility
- EFOP-3.6.2-16-2017-00006/Human Resources Development Operational Programme Grant of the National Research, Development and Innovation Fund of Hungary
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

