Revisiting T-cell adhesion molecules as potential targets for cancer immunotherapy: CD226 and CD2
- PMID: 39349829
- PMCID: PMC11541569
- DOI: 10.1038/s12276-024-01317-9
Revisiting T-cell adhesion molecules as potential targets for cancer immunotherapy: CD226 and CD2
Abstract
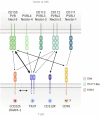
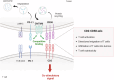
Cancer immunotherapy aims to initiate or amplify immune responses that eliminate cancer cells and create immune memory to prevent relapse. Immune checkpoint inhibitors (ICIs), which target coinhibitory receptors on immune effector cells, such as CTLA-4 and PD-(L)1, have made significant strides in cancer treatment. However, they still face challenges in achieving widespread and durable responses. The effectiveness of anticancer immunity, which is determined by the interplay of coinhibitory and costimulatory signals in tumor-infiltrating immune cells, highlights the potential of costimulatory receptors as key targets for immunotherapy. This review explores our current understanding of the functions of CD2 and CD226, placing a special emphasis on their potential as novel agonist targets for cancer immunotherapy. CD2 and CD226, which are present mainly on T and NK cells, serve important functions in cell adhesion and recognition. These molecules are now recognized for their costimulatory benefits, particularly in the context of overcoming T-cell exhaustion and boosting antitumor responses. The importance of CD226, especially in anti-TIGIT therapy, along with the CD2‒CD58 axis in overcoming resistance to ICI or chimeric antigen receptor (CAR) T-cell therapies provides valuable insights into advancing beyond the current barriers of cancer immunotherapy, underscoring their promise as targets for novel agonist therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Hegde, P. S. & Chen, D. S. Top 10 challenges in cancer immunotherapy. Immunity52, 17–35 (2020). - PubMed
-
- Mueller, D. L., Jenkins, M. K. & Schwartz, R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu. Rev. Immunol.7, 445–480 (1989). - PubMed
-
- Mayes, P. A., Hance, K. W. & Hoos, A. The promise and challenges of immune agonist antibody development in cancer. Nat. Rev. Drug Discov.17, 509–527 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

