CBL-b E3 ligase-mediated neddylation and activation of PARP-1 induce vascular calcification
- PMID: 39349831
- PMCID: PMC11541702
- DOI: 10.1038/s12276-024-01322-y
CBL-b E3 ligase-mediated neddylation and activation of PARP-1 induce vascular calcification
Abstract
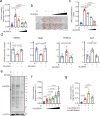
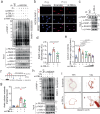
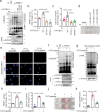
Vascular calcification (VC) refers to the accumulation of mineral deposits on the walls of arteries and veins, and it is closely associated with increased mortality in cardiovascular disease patients, particularly among high-risk patients with diabetes and chronic kidney disease (CKD). Neuronal precursor cell-expressed developmentally downregulated protein 8 (NEDD8) is a ubiquitin-like protein that plays a pivotal role in various cellular functions, primarily through its conjugation to target proteins and subsequent relay of biological signals. However, the role of NEDDylation in VC has not been investigated. In our study, we observed that MLN4924, an inhibitor of the NEDD8-activating E1 enzyme, effectively impedes the progression of VC. LC‒MS/MS analysis revealed that poly(ADP‒ribose) polymerase 1 (PARP-1) is subjected to NEDD8 conjugation, leading to an increase in PARP-1 activity during VC. We subsequently revealed that PARP-1 NEDDylation is mediated by the E3 ligase CBL proto-oncogene B (CBL-b) and is reversed by NEDD8-specific protease 1 (NEDP-1) during VC. Furthermore, the CBL-b C373 peptide effectively mitigated the inactive form of the E3 ligase activity of CBL-b, ultimately preventing VC. These findings provide compelling evidence that the NEDD8-dependent activation of PARP-1 represents a novel mechanism underlying vascular calcification and suggests a promising new therapeutic target for VC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Nicoll, R. & Henein, M. Arterial calcification: a new perspective? Int. J. Cardiol.228, 11–22 (2017). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

