Microbiome-derived antimicrobial peptides show therapeutic activity against the critically important priority pathogen, Acinetobacter baumannii
- PMID: 39349945
- PMCID: PMC11443000
- DOI: 10.1038/s41522-024-00560-2
Microbiome-derived antimicrobial peptides show therapeutic activity against the critically important priority pathogen, Acinetobacter baumannii
Abstract
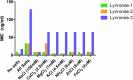
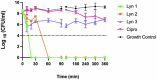
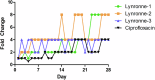
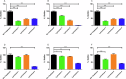
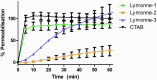
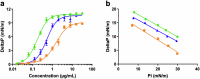
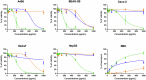
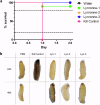
Acinetobacter baumannii is designated by the World Health Organisation as a critical priority pathogen. Previously we discovered antimicrobial peptides (AMPs), namely Lynronne-1, -2 and -3, with efficacy against bacterial pathogens, such as Staphylococcus aureus and Pseudomonas aeruginosa. Here we assessed Lynronne-1, -2 and -3 structure by circular dichroism and efficacy against clinical strains of A. baumannii. All Lynronne AMPs demonstrated alpha-helical secondary structures and had antimicrobial activity towards all tested strains of A. baumannii (Minimum Inhibitory Concentrations 2-128 μg/ml), whilst also having anti-biofilm activity. Lynronne-2 and -3 demonstrated additive effects with amoxicillin and erythromycin, and synergy with gentamicin. The AMPs demonstrated little toxicity towards mammalian cell lines or Galleria mellonella. Fluorescence-based assay data demonstrated that Lynronne-1 and -3 had higher membrane-destabilising action against A. baumannii in comparison with Lynronne-2, which was corroborated by transcriptomic analysis. For the first time, we demonstrate the therapeutic activity of Lynronne AMPs against A. baumannii.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Heidari, H. et al. Molecular analysis of drug-resistant Acinetobacter baumannii isolates by ERIC-PCR. Meta Gene17, 132–135 (2018). - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

