Dysregulation of miRNA expression and excitation in MEF2C autism patient hiPSC-neurons and cerebral organoids
- PMID: 39349966
- PMCID: PMC11919750
- DOI: 10.1038/s41380-024-02761-9
Dysregulation of miRNA expression and excitation in MEF2C autism patient hiPSC-neurons and cerebral organoids
Abstract
MEF2C is a critical transcription factor in neurodevelopment, whose loss-of-function mutation in humans results in MEF2C haploinsufficiency syndrome (MHS), a severe form of autism spectrum disorder (ASD)/intellectual disability (ID). Despite prior animal studies of MEF2C heterozygosity to mimic MHS, MHS-specific mutations have not been investigated previously, particularly in a human context as hiPSCs afford. Here, for the first time, we use patient hiPSC-derived cerebrocortical neurons and cerebral organoids to characterize MHS deficits. Unexpectedly, we found that decreased neurogenesis was accompanied by activation of a micro-(mi)RNA-mediated gliogenesis pathway. We also demonstrate network-level hyperexcitability in MHS neurons, as evidenced by excessive synaptic and extrasynaptic activity contributing to excitatory/inhibitory (E/I) imbalance. Notably, the predominantly extrasynaptic (e)NMDA receptor antagonist, NitroSynapsin, corrects this aberrant electrical activity associated with abnormal phenotypes. During neurodevelopment, MEF2C regulates many ASD-associated gene networks, suggesting that treatment of MHS deficits may possibly help other forms of ASD as well.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare that S.A.L. is an inventor on worldwide patents for the use of memantine, NitroSynapsin, and related aminoadamantane and aminoadamantane nitrate drugs for neurodegenerative and neurodevelopmental disorders. Per Harvard University guidelines, S.A.L. participates in a royalty-sharing agreement with his former institution Boston Children’s Hospital/Harvard Medical School, which licensed the drug memantine (Namenda®) to Forest Laboratories/Actavis/Allergan/AbbVie, Inc. NitroSynapsin (aka EM-036) is licensed to EuMentis Therapeutics, Inc., a biotech in the Boston area for which S.A.L. served as scientific founder. The other authors declare no financial conflicts of interest. All data are available in the main text or the supplementary materials. Ethics approval and consent to participate: All methods were performed in accordance with the relevant guidelines and regulations. Accordingly, the use of human cells was approved by the institutional review boards of the Scintillon Institute and The Scripps Research Institute (TSRI; IRB-19-7428), and informed consent was obtained from all participants or their appropriate legal guardians.
Figures
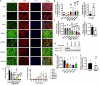







References
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th Ed. American Psychiatric Association; 2013. United States.
-
- Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators, Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. - PubMed

