Facile quantitative diagnostic testing for neutralizing antibodies against Chikungunya virus
- PMID: 39350079
- PMCID: PMC11440707
- DOI: 10.1186/s12879-024-09973-y
Facile quantitative diagnostic testing for neutralizing antibodies against Chikungunya virus
Abstract
Background: Viral neutralization (NT) assays can be used to determine the immune status of patients or assess the potency of candidate vaccines or therapeutic monoclonal antibodies (mAbs). Focus reduction neutralization test (FRNT) is a conventional neutralization test (cVNT) with superior specificity for measurement of neutralizing antibodies against a specific virus. Unfortunately, the application of FRNT to the chikungunya virus (CHIKV) involves a highly pathogenic bio-agent requiring biosafety level 3 (BSL3) facilities, which inevitably imposes high costs and limits accessibility. In this study, we evaluated a safe surrogate virus neutralization test (sVNT) that uses novel CHIKV replicon particles (VRPs) expressing eGFP and luciferase (Luc) to enable the rapid detection and quantification of neutralizing activity in clinical human serum samples.
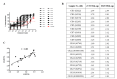
Methods: This unmatched case-control validation study used serum samples from laboratory-confirmed cases of CHIKV (n = 19), dengue virus (DENV; n = 9), Japanese encephalitis virus (JEV; n = 5), and normal individuals (n = 20). We evaluated the effectiveness of sVNT, based on mosquito cell-derived CHIK VRPs (mos-CHIK VRPs), in detecting (eGFP) and quantifying (Luc) neutralizing activity, considering specificity, sensitivity, and reproducibility. We conducted correlation analysis between the proposed rapid method (20 h) versus FRNT assay (72 h). We also investigated the correlation between sVNT and FRNT in NT titrations in terms of Pearson's correlation coefficient (r) and sigmoidal curve fitting.
Results: In NT screening assays, sVNT-eGFP screening achieved sensitivity and specificity of 100%. In quantitative neutralization assays, we observed a Pearson's correlation coefficient of 0.83 for NT50 values between sVNT-Luc and FRNT.
Conclusions: Facile VRP-based sVNT within 24 h proved highly reliable in the identification and quantification of neutralizing activity against CHIKV in clinical serum samples.
Keywords: Chikungunya virus; Diagnostics; Neutralizing antibodies; Surrogate virus neutralization test; Surveillance; Vaccine; Virus-like replicon particle.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Velasco JM, Valderama MT, Lopez MN, Chua D Jr., Latog R 2nd, Roque V Jr., Corpuz J, Klungthong C, Rodpradit P, Hussem K, et al. Chikungunya Virus infections among patients with Dengue-Like illness at a Tertiary Care Hospital in the Philippines, 2012–2013. Am J Trop Med Hyg. 2015;93(6):1318–24. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

