Long-term antibody response after the third dose of inactivated SARS-CoV-2 vaccine in MASLD patients
- PMID: 39350092
- PMCID: PMC11441169
- DOI: 10.1186/s12876-024-03402-9
Long-term antibody response after the third dose of inactivated SARS-CoV-2 vaccine in MASLD patients
Abstract
Background: Metabolic dysfunction-associated steatotic liver disease (MASLD) patients are at an elevated risk of developing severe coronavirus disease 2019 (COVID-19). The objective of this study was to assess antibody responses and safety profiles six months after the third dose of the inactivated acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine in MASLD patients.
Methods: This study included MASLD patients and healthy volunteers without a history of SARS-CoV-2 infection. Blood samples were collected six months after receiving the third dose of the inactivated vaccine to measure the levels of neutralizing antibodies (NAbs) and anti-spike IgG antibodies against SARS-CoV-2.
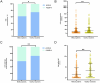
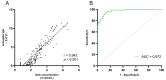
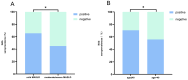
Results: A total of 335 participants (214 MASLD patients and 121 healthy volunteers) were enrolled. The seroprevalence of NAb was 61.7% (132 of 214) in MASLD patients and 74.4% (90 of 121) in healthy volunteers, which was a significant difference (p = 0.018). Statistically significant differences in IgG seroprevalence were also observed between MASLD patients and healthy volunteers (p = 0.004). Multivariate analysis demonstrated that the severity of MASLD (OR, 2.97; 95% CI, 1.32-6.68; p = 0.009) and age (OR, 1.03; 95% CI, 1.01-1.06; p = 0.004) were independent risk factors for NAb negativity in MASLD patients. Moderate/severe MASLD patients had a lower NAb seroprevalence than mild MASLD patients (45.0% vs. 65.5%, p = 0.016).
Conclusion: Lower antibody responses were observed in MASLD patients six months after their third dose of the inactivated vaccine than in healthy volunteers, providing further assistance in monitoring patients who are more vulnerable to hypo-responsiveness to SARS-CoV-2 vaccines.
Keywords: Antibody response; Metabolic dysfunction-associated steatotic liver disease; SARS-CoV-2; Vaccine.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting.Front Immunol. 2021 Dec 22;12:802858. doi: 10.3389/fimmu.2021.802858. eCollection 2021. Front Immunol. 2021. PMID: 35003131 Free PMC article.
-
Long-Term Kinetics of SARS-CoV-2 Antibodies and Impact of Inactivated Vaccine on SARS-CoV-2 Antibodies Based on a COVID-19 Patients Cohort.Front Immunol. 2022 Jan 27;13:829665. doi: 10.3389/fimmu.2022.829665. eCollection 2022. Front Immunol. 2022. PMID: 35154152 Free PMC article.
-
Response and Duration of Serum Anti-SARS-CoV-2 Antibodies After Inactivated Vaccination Within 160 Days.Front Immunol. 2021 Dec 23;12:786554. doi: 10.3389/fimmu.2021.786554. eCollection 2021. Front Immunol. 2021. PMID: 35003104 Free PMC article.
-
Clinical and laboratory features in health care volunteers with inactivated SARS-CoV-2 vaccination.Turk J Med Sci. 2023 May 25;53(5):1185-1193. doi: 10.55730/1300-0144.5684. eCollection 2023. Turk J Med Sci. 2023. PMID: 38813035 Free PMC article.
-
Risk of Serious Bacterial and Non-Bacterial Infections in People With MASLD.Liver Int. 2025 Apr;45(4):e70059. doi: 10.1111/liv.70059. Liver Int. 2025. PMID: 40072231 Free PMC article. Review.
Cited by
-
Post-COVID-19 Pandemic Sequelae in Liver Diseases.Life (Basel). 2025 Mar 4;15(3):403. doi: 10.3390/life15030403. Life (Basel). 2025. PMID: 40141748 Free PMC article. Review.
References
-
- World Health Organization, Coronavirus (COVID-19). wwwwhoint. (2023). Accessed 8 Aug 2024.
-
- Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47-S64. - PubMed
-
- Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341–50. - PubMed
-
- Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(9):851–61. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

