Gastrointestinal adverse events of metformin treatment in patients with type 2 diabetes mellitus: a systematic review and meta-analysis with meta-regression of observational studies
- PMID: 39350158
- PMCID: PMC11440709
- DOI: 10.1186/s12902-024-01727-w
Gastrointestinal adverse events of metformin treatment in patients with type 2 diabetes mellitus: a systematic review and meta-analysis with meta-regression of observational studies
Abstract
Introduction: Metformin is the most prescribed medication for type 2 diabetes mellitus (T2DM); there is a well-established link with the elevated incidence of gastrointestinal (GI) adverse events (AE) limiting its administration or intensification.
Objectives: The objective of this systematic review and meta-analysis of observational studies was to evaluate the pooled incidence of GI AE related to metformin use in patients with T2DM.
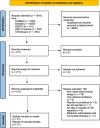
Materials and methods: PUB MED/CINAHL/Web of Science/Scopus were searched from database inception until 29.07.2024 for observational studies in English describing the frequency of GI AE in patients with T2DM treated with metformin. Random-effects meta-analyses were used to derive effect sizes: event rates.
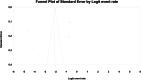
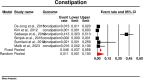
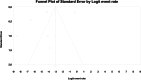
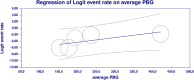
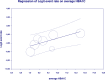
Results: From 7019 publications, we identified 211 potentially eligible full-text articles. Ultimately, 21 observational studies were included in the meta-analysis. The prevalence of GI AE was as follows: diarrhea 6.9% (95% CI: 0.038-0.123), bloating 6,2% (95% CI: 0.020-0.177), abdominal pain 5,3% (95% CI: 0.003-0.529), vomiting 2.4% (95%: CI 0.007-0.075), constipation 1.1% (95%: CI 0.001-0.100). The incidence of bloating (coefficient -4.46; p < 0.001), diarrhea (coefficient -1.17; p = 0.0951) abdominal pain (coefficient -2.80; p = 0.001), constipation (coefficient -5.78; p = 0.0014) and vomiting (coefficient -2.47; p < 0.001) were lower for extended release (XR) metformin than metformin immediate release (IR) formulation.
Conclusions: This study highlights the prevalence of GI AE in patients receiving metformin, with a diarrhea predominance, followed by bloating, diarrhea, abdominal pain, constipation, and vomiting. The incidence is lower in patients administered with XR metformin.
Trial registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021289975 , identifier CRD42021289975.
Keywords: Diarrhea; Dose; Formulation; Gastrointestinal adverse events; Meta-analysis; Metformin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures















References
-
- Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60(9):1566–76. 10.1007/s00125-017-4318-z. - PubMed
-
- IDF Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes: recommendations for standard, comprehensive, and minimal care. Diabet Med. 2006;23(6):579–93. 10.1111/j.1464-5491.2006.01918.x. - PubMed
-
- Magliano DJ, Boyko EJ, IDF Diabetes Atlas 10th edition scientific committee. IDF Diabetes Atlas. 10th ed. International Diabetes Federation; 2021. Accessed October 4, 2023. http://www.ncbi.nlm.nih.gov/books/NBK581934/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

