Associations of combined accelerated biological aging and genetic susceptibility with incident dementia: a prospective study in the UK Biobank
- PMID: 39350213
- PMCID: PMC11443929
- DOI: 10.1186/s12916-024-03640-4
Associations of combined accelerated biological aging and genetic susceptibility with incident dementia: a prospective study in the UK Biobank
Abstract
Background: Accelerated biological aging has been verified to be a critical risk factor for a number of age-related diseases, but its role in dementia remained unclear. Whether it modified the effects of genetic factors was also unknown. This study evaluated the associations between accelerated biological aging and dementia and the moderating role of accelerated biological aging in the genetic susceptibility to the disease.
Methods: We included 200,731 participants in the UK biobank. Nine clinical blood biomarkers and chronological age were used to calculate Phenotypic age acceleration (PhenoAgeAccel), which is a novel indicator for accelerated biological aging. The associations of PhenoAgeAccel with dementia, both young-onset and late-onset dementia, were assessed by Cox proportional hazard models. Apolipoprotein E (APOE) alleles and polygenic risk scores (PRS) were used to evaluate the genetic risk of dementia. The interactions between genetic susceptibility and biological aging were tested on both multiplicative and additive scales.
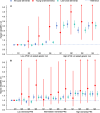
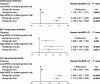
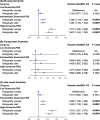
Results: These findings showed individuals who were in the highest quartile of PhenoAgeAccel had a higher risk with incidence of dementia compared to individuals in the lowest quartile of PhenoAgeAccel (HR: 1.145 (95% CI: 1.050, 1.249)). Individuals with biologically older had a higher risk of dementia than individuals with biologically younger (HR: 1.069 (95% CI: 1.004, 1.138)). Furthermore, compared to individuals with biologically younger and low APOE ε4-related genetic risk, individuals with biologically younger and high APOE ε4-related genetic risk (HR:3.048 (95% CI: 2.811, 3.305)) had a higher risk of dementia than individuals with biologically older and high APOE ε4-related genetic risk (HR: 2.765 (95% CI: 2.523, 3.029)). Meanwhile, referring to low dementia PRS and biologically younger, the risk of dementia increased by 72.7% (HR: 1.727 (95% CI: 1.538, 1.939) in the biologically younger and high PRS group and 58.7% (HR: 1.587 (95% CI: 1.404, 1.793) in the biologically older and high PRS group, respectively. The negative interactions between PhenoAgeAccel with APOE ε4 and PRS were also tested on the additive scale.
Conclusions: Accelerated biological aging could bring the extra risk of dementia but attenuate the effects of genetic risk on dementia. These findings provide insights for precise prevention and intervention of dementia.
Keywords: Accelerated biological aging; Apolipoprotein E; Attributable proportion; Dementia; Polygenetic risk score; Relative excess risk; UK Biobank.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Deckers K, van Boxtel MP, Schiepers OJ, de Vugt M, Munoz Sanchez JL, Anstey KJ, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30(3):234–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

