Intravitreal faricimab for treatment naïve patients with neovascular age-related macular degeneration: a real-world prospective study
- PMID: 39350227
- PMCID: PMC11441120
- DOI: 10.1186/s40942-024-00586-w
Intravitreal faricimab for treatment naïve patients with neovascular age-related macular degeneration: a real-world prospective study
Abstract
Background: Intravitreal faricimab, a bispecific antibody targeting both angiopoietin-2 (Ang-2) and vascular endothelial growth factor-A (VEGF-A), was recently introduced for the treatment of neovascular age-related macular degeneration (nAMD), diabetic macular oedema and cystoid macular oedema secondary to retinal vein occlusion. The aim of our study was to assess the efficacy, safety and durability of intravitreal faricimab in a real-world cohort of treatment-naïve patients with nAMD.
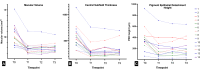
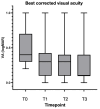
Methods: Single-centre, prospective cohort study of 21 eyes from 19 treatment-naïve nAMD patients who were treated with intravitreal faricimab from October 2022 to April 2024. Patients underwent a loading dose (LD) of 4 monthly faricimab injections followed by a treat-and-extend regimen. Primary outcomes included best-corrected visual acuity (BCVA) and structural parameters from spectral-domain optical coherence tomography (SD-OCT). Secondary outcomes included the proportion of eyes achieving a dry macula, maximal fluid-free interval and intended interval at last follow-up.
Results: The study included 21 eyes of 19 patients (mean age 83.1 years). After LD, 93.3% of eyes achieved a dry macular SD-OCT scan within a median time of 8 weeks. At the first extension, 53% of eyes remained dry, while 47% showed fluid recurrence. Long-term analysis (n = 14) revealed significant reductions in macular volume (MV), central subfield thickness (CST), and pigment epithelial detachment (PED) height over a median follow-up of 64.9 weeks, with sustained visual and anatomical improvements. Median BCVA, CST, and MV at the final follow-up were significantly improved from baseline (p < 0.01). The intended interval between injections was ≥ 12 weeks in 42.86% of eyes. No cases of intraocular inflammation were observed, although 10% experienced retinal pigment epithelial tears.
Conclusions: Intravitreal faricimab demonstrated favourable efficacy, safety, and durability outcomes in a real-world cohort of treatment-naïve nAMD patients.
Keywords: Age-related macular degeneration; Anti-angiopoietin 2; Anti-vascular endothelial growth factor; Faricimab; Treat-and-extend.
© 2024. The Author(s).
Conflict of interest statement
GG provided consulting and/or speaker services for Abbvie, Apellis, Bayer and Roche. MM provided consulting and/or speaker services for Abbvie, Apellis, Bayer, Endogena Inc, Novartis, Roche and holds equity of Endogena Inc.
Figures

References
-
- Fleckenstein M, Schmitz-Valckenberg S, Chakravarthy U. Age-related Macular Degeneration: a review. JAMA. 2024;331(2):147. - PubMed
-
- Vabysmo Public Summary SwissPAR [Internet]. Swissmedic. 2022. https://www.swissmedic.ch/swissmedic/en/home/about-us/publications/publi...
-
- Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729–40. - PubMed
-
- Khanani AM, Kotecha A, Chang A, Chen SJ, Chen Y, Guymer R et al. TENAYA and LUCERNE. Ophthalmology. 2024;S0161642024001349.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

