Engineered liver-derived decellularized extracellular matrix-based three-dimensional tumor constructs for enhanced drug screening efficiency
- PMID: 39350956
- PMCID: PMC11441757
- DOI: 10.1093/rb/rbae113
Engineered liver-derived decellularized extracellular matrix-based three-dimensional tumor constructs for enhanced drug screening efficiency
Abstract
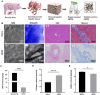
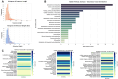
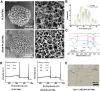
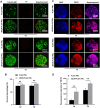
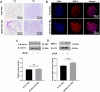
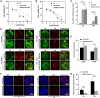
The decellularized extracellular matrix (dECM) has emerged as an effective medium for replicating the in vivo-like conditions of the tumor microenvironment (TME), thus enhancing the screening accuracy of chemotherapeutic agents. However, recent dECM-based tumor models have exhibited challenges such as uncontrollable morphology and diminished cell viability, hindering the precise evaluation of chemotherapeutic efficacy. Herein, we utilized a tailor-made microfluidic approach to encapsulate dECM from porcine liver in highly poly(lactic-co-glycolic acid) (PLGA) porous microspheres (dECM-PLGA PMs) to engineer a three-dimensional (3D) tumor model. These dECM-PLGA PMs-based microtumors exhibited significant promotion of hepatoma carcinoma cells (HepG2) proliferation compared to PLGA PMs alone, since the infusion of extracellular matrix (ECM) microfibers and biomolecular constituents within the PMs. Proteomic analysis of the dECM further revealed the potential effects of these bioactive fragments embedded in the PMs. Notably, dECM-PLGA PMs-based microtissues effectively replicated the drug resistance traits of tumors, showing pronounced disparities in half-maximal inhibitory concentration (IC50) values, which could correspond with certain aspects of the TME. Collectively, these dECM-PLGA PMs substantially surmounted the prevalent challenges of unregulated microstructure and suboptimal cell viability in conventional 3D tumor models. They also offer a sustainable and scalable platform for drug testing, holding promise for future pharmaceutical evaluations.
Keywords: cancer; decellularized extracellular matrix; microfluidics; preclinical drug screening; three-dimensional tumor model.
© The Author(s) 2024. Published by Oxford University Press.
Figures


References
-
- Wakefield L, Agarwal S, Tanner K.. Preclinical models for drug discovery for metastatic disease. Cell 2023;186:1792–813. - PubMed
-
- Thoma CR, Zimmermann M, Agarkova I, Kelm JM, Krek W.. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Deliv Rev 2014;69–70:29–41. - PubMed
-
- Brancato V, Oliveira JM, Correlo VM, Reis RL, Kundu SC.. Could 3D models of cancer enhance drug screening? Biomaterials 2020;232:119744. - PubMed
-
- Ma JN, Dai LS, Yu JB, Cao H, Bao YM, Hu JJ, Zhou LH, Yang JQ, Sofia A, Chen HW, Wu F, Xie ZK, Qian WQ, Zhan RY.. Tumor microenvironment targeting system for glioma treatment via fusion cell membrane coating nanotechnology. Biomaterials 2023;295:122026. - PubMed
LinkOut - more resources
Full Text Sources

