Aconitate decarboxylase 1 mediates the acute airway inflammatory response to environmental exposures
- PMID: 39351225
- PMCID: PMC11439662
- DOI: 10.3389/fimmu.2024.1432334
Aconitate decarboxylase 1 mediates the acute airway inflammatory response to environmental exposures
Abstract
Background: Environmental lipopolysaccharide (LPS) and microbial component-enriched organic dusts cause significant lung disease. These environmental exposures induce the recruitment and activation of distinct lung monocyte/macrophage subpopulations involved in disease pathogenesis. Aconitate decarboxylase 1 (Acod1) was one of the most upregulated genes following LPS (vs. saline) exposure of murine whole lungs with transcriptomic profiling of sorted lung monocyte/macrophage subpopulations also highlighting its significance. Given monocyte/macrophage activation can be tightly linked to metabolism, the objective of these studies was to determine the role of the immunometabolic regulator ACOD1 in environmental exposure-induced lung inflammation.
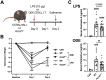
Methods: Wild-type (WT) mice were intratracheally (i.t.) instilled with 10 μg of LPS or saline. Whole lungs were profiled using bulk RNA sequencing or sorted to isolate monocyte/macrophage subpopulations. Sorted subpopulations were then characterized transcriptomically using a NanoString innate immunity multiplex array 48 h post-exposure. Next, WT and Acod1-/- mice were instilled with LPS, 25% organic dust extract (ODE), or saline, whereupon serum, bronchoalveolar lavage fluid (BALF), and lung tissues were collected. BALF metabolites of the tricarboxylic acid (TCA) cycle were quantified by mass spectrometry. Cytokines/chemokines and tissue remodeling mediators were quantitated by ELISA. Lung immune cells were characterized by flow cytometry. Invasive lung function testing was performed 3 h post-LPS with WT and Acod1-/- mice.
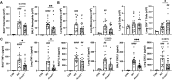
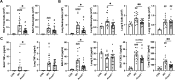
Results: Acod1-/- mice treated with LPS demonstrated decreased BALF levels of itaconate, TCA cycle reprogramming, decreased BALF neutrophils, increased lung CD4+ T cells, decreased BALF and lung levels of TNF-α, and decreased BALF CXCL1 compared to WT animals. In comparison, Acod1-/- mice treated with ODE demonstrated decreased serum pentraxin-2, BALF levels of itaconate, lung total cell, neutrophil, monocyte, and B-cell infiltrates with decreased BALF levels of TNF-α and IL-6 and decreased lung CXCL1 vs. WT animals. Mediators of tissue remodeling (TIMP1, MMP-8, MMP-9) were also decreased in the LPS-exposed Acod1-/- mice, with MMP-9 also reduced in ODE-exposed Acod1-/- mice. Lung function assessments demonstrated a blunted response to LPS-induced airway hyperresponsiveness in Acod1-/- animals.
Conclusion: Acod1 is robustly upregulated in the lungs following LPS exposure and encodes a key immunometabolic regulator. ACOD1 mediates the proinflammatory response to acute inhaled environmental LPS and organic dust exposure-induced lung inflammation.
Keywords: ACOD1; endotoxin; environmental health; immunometabolism; inhalation; macrophages; organic dust.
Copyright © 2024 Schwab, Nelson, Gleason, Schanze, Wyatt, Shinde, Xiao, Thomas, Guda, Bailey, Kielian, Thiele and Poole.
Conflict of interest statement
JP has received research reagent from AstraZeneca (no monies) and has been a site investigator for allergy and asthma clinical studies for Takeda, GlaxoSmithKline, Regeneron, Areteia, and AstraZeneca (no monies). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Prüss-Ustün A, Wolf J, Corvalán C, Neville T, Bos R, Neira M, et al. Diseases due to unhealthy environments: an updated estimate of the global burden of disease attributable to environmental determinants of health. J Public Health (Oxf). (2017) 39:464–75. doi: 10.1093/pubmed/fdw085 - DOI - PMC - PubMed
-
- Rolph CA, Gwyther CL, Tyrrel SF, Nasir ZA, Drew GH, Jackson SK, et al. Sources of airborne endotoxins in ambient air and exposure of nearby communities—A review. Atmosphere. (2018) 9:375. doi: 10.3390/atmos9100375 - DOI
-
- Park S, Allen RJ, Lim CH. A likely increase in fine particulate matter and premature mortality under future climate change. Air Quality Atmosphere Health. (2020) 13:143–51. doi: 10.1007/s11869-019-00785-7 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

