Impact of mAb-FcRn affinity on IgG transcytosis across human well-differentiated airway epithelium
- PMID: 39351230
- PMCID: PMC11439726
- DOI: 10.3389/fimmu.2024.1371156
Impact of mAb-FcRn affinity on IgG transcytosis across human well-differentiated airway epithelium
Abstract
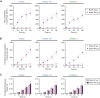
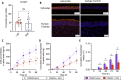
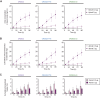
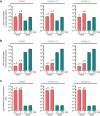
Effective treatment and immunoprophylaxis of viral respiratory infections with neutralizing monoclonal antibodies (mAbs) require maintaining inhibitory concentrations of mAbs at the airway surface. While engineered mAbs with increased affinity to the neonatal Fc receptor (FcRn) are increasingly employed, little is known how increased affinity of Fc to FcRn influences basal-to-apical transepithelial transport (transcytosis) of mAbs across the airway epithelium. To investigate this, we utilized a model of well-differentiated human airway epithelium (WD-HAE) that exhibited robust FcRn expression, and measured the transepithelial transport of a mAb against SARS-CoV-2 Spike protein (CR3022) with either wildtype IgG1-Fc or Fc modified with YTE or LS mutations known to increase affinity for FcRn. Despite the marked differences in the affinity of these CR3022 variants for FcRn, we did not find substantial differences in basal-to-apical transport reflective of systemic dosing, or apical-to-basal transport reflective of inhaled dosing, compared to the transport of wildtype IgG1-Fc. These results suggest increasing FcRn affinity may only have limited influence over transcytosis rates of systemically dosed mAbs across the human airway epithelium over short time scales. Over longer time scales, the elevated circulating levels of mAbs with greater FcRn affinity, due to more effective FcRn-mediated recycling, may better resupply mAb into the respiratory tract, leading to more effective extended immunoprophylaxis.
Keywords: FcRn; airway; lung; monoclonal Abs; respiratory virus; transcytosis.
Copyright © 2024 Togami, Wolf, Olson, Card, Shen, Schaefer, Okuda, Zeitlin, Pauly, Whaley, Pickles and Lai.
Conflict of interest statement
Authors LZ, MP, and KW were employed by the company ZabBio. Author SL is founder of Mucommune, LLC and currently serves as its interim CEO. Author SL is also founder of Inhalon Biopharma, Inc, and currently serves as its CSO as well as on its Board of Director and Scientific Advisory Board. Author SL has equity interests in both Mucommune and Inhalon Biopharma; Author SL’s relationships with Mucommune and Inhalon are subject to certain restrictions under university policy. The terms of these arrangements are managed by UNC-CH in accordance with its conflict-of-interest policies. Author SL was inventor of intellectual property that has been licensed to Inhalon Biopharma. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Sims AC, Baric RS, Yount B, Burkett SE, Collins PL, Pickles RJ. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J Virol. (2005) 79:15511–24. doi: 10.1128/jvi.79.24.15511-15524.2005 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

