Testing home-based exercise strategies in underserved minority cancer patients undergoing chemotherapy (THRIVE) trial: a study protocol
- PMID: 39351353
- PMCID: PMC11439870
- DOI: 10.3389/fonc.2024.1427046
Testing home-based exercise strategies in underserved minority cancer patients undergoing chemotherapy (THRIVE) trial: a study protocol
Abstract
Background: Higher rates of physical inactivity and comorbid conditions are reported in Hispanic/Latinx and Black cancer patients receiving chemotherapy compared to their White counterparts. Despite the beneficial effect of exercise training for cancer patients, rates of participation in exercise oncology clinical trials are low among disadvantaged and racial and ethnic minority groups. Here, we will examine the effect of an exercise intervention using a novel, accessible, and cost-effective home-based exercise approach among Hispanic/Latinx and Black cancer patients receiving chemotherapy on exercise participation and cardiovascular disease risk.
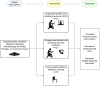
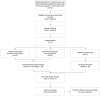
Methods: The THRIVE trial is an 8-month prospective, three-arm study of 45 patients who are randomized in a 1:1:1 fashion to a supervised exercise intervention (SUP), unsupervised exercise (UNSUP), or an attention control (AC) group. Eligible patients include those with breast, colorectal, or prostate cancer, who are sedentary, overweight or obese, self-identify as Hispanic/Latinx or Black, and plan to receive chemotherapy. Patients randomized to the SUP group participate in a home-based 16-week periodized aerobic and resistance exercise program performed three days per week, supervised through video conference technology. Patients randomized to the UNSUP group participate in an unsupervised 16-week, telehealth-based, periodized aerobic and resistance exercise program performed three days per week using the same exercise prescription parameters as the SUP group. Patients randomized to the AC group receive a 16-week home-based stretching program. The primary outcome is changes in minutes of physical activity assessed by 7-day accelerometry at post-intervention. Secondary outcomes include cardiovascular risk factors, patient-reported outcomes, and physical function. Outcome measures are tested at baseline, post-intervention at month 4, and after a non-intervention follow-up period at month 8.
Discussion: The THRIVE trial is the first study to employ a novel and potentially achievable exercise intervention for a minority population receiving chemotherapy. In addition, this study utilizes an intervention approach to investigate the biological and behavioral mechanisms underlying exercise participation in these cancer patients. Results will guide and inform large randomized controlled trials to test the effect of home-based exercise on treatment outcomes and comorbid disease risk in minority patients with cancer undergoing chemotherapy.
Clinical trial registration: https://classic.clinicaltrials.gov/ct2/show/NCT05327452, identifier (NCT#05327452).
Keywords: cancer; cardiovascular risk factors; exercise participation; home-based exercise; racial and ethnic minorities.
Copyright © 2024 Yan, Gonzalo-Encabo, Wilson, Christopher, Cannon, Kang, Gardiner, Perez, Norris, Gundersen, Hayman, Freedman, Rebbeck, Shi and Dieli-Conwright.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

