Evaluation of HER2 immunohistochemistry expression in non-standard solid tumors from a Single-Institution Prospective Cohort
- PMID: 39351438
- PMCID: PMC11438559
- DOI: 10.37349/etat.2024.00265
Evaluation of HER2 immunohistochemistry expression in non-standard solid tumors from a Single-Institution Prospective Cohort
Abstract
Aim: Human epidermal growth factor receptor-2 (HER2) is a well-established prognostic and predictive biomarker. It is an FDA-approved therapeutic target for HER2 positive breast, gastroesophageal, and more recently, lung and colon cancers. It is an emerging biomarker in biliary tract, bladder, cervical, endometrial, ovarian, and pancreatic cancers. The emergence of new indications warrants further characterization of HER2 expression in diverse cancer populations. This study investigated HER2 expression in solid tumour samples and the feasibility of obtaining these results.
Methods: Prospective consent was obtained at a Canadian tertiary academic cancer center from adult oncology patients who were referred for molecular genetic testing of malignant tissue samples. Standard HER2-targeted malignancies were considered breast and gastroesophageal, and were excluded from this study. Between July 2020 and November 2023, 499 samples of solid tumors underwent immunohistochemistry (IHC) HER2 staining. A median turnaround time (TAT) of 14 days would be considered feasible for clinical decision making.
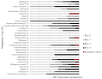
Results: The mean age (± SD) of participants was 67 ± 12.5 years, with 270 (54%) male and 229 (46%) female. HER2 protein expression was measured in 42 unique cancer types. IHC levels of 0, 1+, 2+, and 3+ were reported and were 43%, 12%, 35%, and 10% of all analyzable samples respectively (tissue inadequate in 3% of samples). The median TAT for HER2 expression results from time of request to result in release was 18 (interquartile range, 11 to 30) days.
Conclusions: HER2 protein expression varies widely between different cancer types. TAT for HER2 IHC results was a median of 18 days, which is close to our feasibility cut-off.
Keywords: HER2; IHC; biomarker; solid tumors.
© The Author(s) 2024.
Conflict of interest statement
DB has accepted honoraria and/or speaking fees from Astra Zeneca, Amgen, Bristol-Myers Squibb, Takeda, Bayer, Guardant, Roche, Janssen and Merck. The rest of the authors have no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
