Coadministration of isavuconazole and sirolimus in allogeneic hematopoietic stem cell transplant recipients
- PMID: 39351448
- PMCID: PMC11440545
- DOI: 10.1177/20499361241252539
Coadministration of isavuconazole and sirolimus in allogeneic hematopoietic stem cell transplant recipients
Abstract
Background: Invasive fungal infections (IFIs) represent a major cause of morbidity among allogeneic hematopoietic stem cell transplantation (allo-HSCT). Isavuconazole (ISA) is a broad-spectrum triazole with favorable safety profile.
Objectives and design: Herein, we evaluate the real life coadministration of ISA and sirolimus in allo-HSCT recipients in a single-center retrospective analysis, describing clinical efficacy, safety, and therapeutic drug monitoring (TDM) of both drugs.
Methods: All consecutive allo-HSCT recipients who received the coadministration of ISA and sirolimus for at least 2 weeks between July 2017 and December 2022 were included in this retrospective analysis. TDM was longitudinally performed during treatment. IFIs were classified according to the revised European Organization for Research and Treatment of Cancer/Mycoses Study Group consensus criteria.
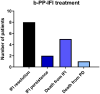
Results: A total of 51 recipients were included in the analysis. A total of 17 patients received ISA as continuous antifungal treatment for IFI diagnosed before transplant: one patient experienced a probable invasive pulmonary aspergillosis, and one patient switched from ISA to liposomal amphotericin B for a possible IFI. A total of 34 patients started ISA as antifungal therapy for IFI diagnosed after transplant. Sixteen of 34 were treated for a proven/probable breakthrough IFI during mold-active prophylaxis: 6/16 patients died for IFI after a median of 51 days of ISA. Eighteen of 34 started ISA as empirical therapy for a possible IFI: 15/18 patients were alive with resolution of infection after 6 weeks, 1 died for disease progression, and 2 had empirically changed antifungal therapy due to pneumonia progression. Clinical and radiological response rate was 68% after 90 days from IFI diagnosis. No toxicities related to drug-drug interaction have been registered in patients reaching concomitant therapeutic levels of ISA and sirolimus.
Conclusion: The coadministration of ISA and sirolimus was safe and feasible in this cohort, confirming favorable clinical efficacy in patients with multiple-drug coadministration.
Keywords: IFI; TDM; allogeneic transplantation; isavuconazole; sirolimus.
© The Author(s), 2024.
Figures


References
-
- Styczynski J, Tridello G, Koster L, et al.. Decrease of lethal infectious complications in the context of causes of death (COD) after hematopoietic cell transplantation: COD-2 and COD-1 study of the Infectious Diseases Working Party EBMT. Bone Marrow Transplant 2023; 58: 881–892. - PubMed
-
- Neofytos D, Horn D, Anaissie E, et al.. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis 2009; 48: 265–273. - PubMed
-
- Lindsay J, Kerridge I, Wilcox L, et al.. Infection-related mortality in adults and children undergoing allogeneic hematopoietic cell transplantation: an Australian Registry Report. Transplant Cell Ther 2021; 27: 798.e1–798.e10. - PubMed
-
- Kontoyiannis DP, Marr KA, Park BJ, et al.. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the transplant-associated infection surveillance network (TRANSNET) database. Clin Infect Dis 2010; 50: 1091–1100. - PubMed
-
- Girmenia C, Barosi G, Piciocchi A, et al.. Primary prophylaxis of invasive fungal diseases in allogeneic stem cell transplantation: revised recommendations from a consensus process by Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant 2014; 20: 1080–1088. - PubMed
LinkOut - more resources
Full Text Sources

