Case Series: ATRX Variants in Four Patients with Metastatic Pheochromocytoma
- PMID: 39351526
- PMCID: PMC11439680
- DOI: 10.3389/fendo.2024.1399847
Case Series: ATRX Variants in Four Patients with Metastatic Pheochromocytoma
Erratum in
-
Correction: Case series: ATRX variants in four patients with metastatic pheochromocytoma.Front Endocrinol (Lausanne). 2025 Nov 14;16:1736207. doi: 10.3389/fendo.2025.1736207. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 41323964 Free PMC article.
Abstract
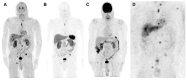
Few reports have highlighted the rare presence of somatic ATRX variants in clinically aggressive, metastatic pheochromocytoma/paraganglioma (PCC/PGL); however, none have addressed detailed clinical presentation (including biochemistry and imaging) and management of these patients. Here, we address these clinical features and management based on four PCC patients with somatic ATRX variants from our National Institutes of Health PCC/PGL cohort. A total of 192 patients underwent exome sequencing (germline, somatic, or both), and four males were found to have somatic ATRX variants (with additional somatic VHL and FH oncogenic variants in patients 2 and 4, respectively). Per-lesion and per-patient comparisons were performed among functional imaging scans performed at the NIH. Biochemical phenotype and response to systemic treatment were evaluated. This mini-series supports prior studies showing aggressive/metastatic PCC in patients with somatic ATRX variants, as all developed widespread metastatic disease. All four PCC patients presented with noradrenergic biochemical phenotype, and some with significant elevation in 3-methoxytyramine. 18F-FDOPA PET/CT was found to be the superior functional imaging modality, with 100% lesion detection rate when compared to that of 68Ga-DOTATATE, 18F-FDG, 18F-FDA, and 123I-MIBG scans. While patients did not respond to chemotherapy or tyrosine kinase inhibitors, they responded to targeted radiotherapy using high-specific-activity 131I-MIBG (Azedra®) or 177Lu-DOTATATE (Lutathera®).
Keywords: ATRX; case report; imaging; pheochromocytoma; prognosis.
Copyright © 2024 Cortez, Kuo, Jha, Patel, Carrasquillo, Prodanov, Charles, Talvacchio, Derkyi, Lin, Taïeb, Del Rivero and Pacak.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, Milosevic D, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. (2012) 48:1739–49. doi: 10.1016/j.ejca.2011.07.016 - DOI - PMC - PubMed
-
- Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, et al. Clinical risk factors for Malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. (2011) 96:717–25. doi: 10.1210/jc.2010-1946 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

