New Bacterial Aryl Sulfotransferases: Effective Tools for Sulfation of Polyphenols
- PMID: 39351615
- PMCID: PMC11468790
- DOI: 10.1021/acs.jafc.4c06771
New Bacterial Aryl Sulfotransferases: Effective Tools for Sulfation of Polyphenols
Abstract
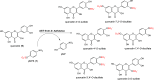
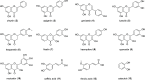
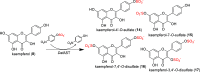
The preparation of pure metabolites of bioactive compounds, particularly (poly)phenols, is essential for the accurate determination of their pharmacological profiles in vivo. Since the extraction of these metabolites from biological material is tedious and impractical, they can be synthesized enzymatically in vitro by bacterial PAPS-independent aryl sulfotransferases (ASTs). However, only a few ASTs have been studied and used for (poly)phenol sulfation. This study introduces new fully characterized recombinant ASTs selected according to their similarity to the previously characterized ASTs. These enzymes, produced in Escherichia coli, were purified, biochemically characterized, and screened for the sulfation of nine flavonoids and two phenolic acids using p-nitrophenyl sulfate. All tested compounds were proved to be substrates for the new ASTs, with kaempferol and luteolin being the best converted acceptors. ASTs from Desulfofalx alkaliphile (DalAST) and Campylobacter fetus (CfAST) showed the highest efficiency in the sulfation of tested polyphenols. To demonstrate the efficiency of the present sulfation approach, a series of new authentic metabolite standards, regioisomers of kaempferol sulfate, were enzymatically produced, isolated, and structurally characterized.
Keywords: aryl sulfotransferase; enzymatic sulfation; kaempferol sulfate; metabolite; polyphenol.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Sak K.Anticancer Action of Sulfated Flavonoids as Phase II Metabolites. In Food Bioconversion; Grumezescu A. M., Holban A. M., Eds.; Academic Press Ltd-Elsevier Science Ltd, 2017; Vol. 2, pp 207–236
-
- Roubalová L.; Purchartová K.; Papoušková B.; Vacek J.; Křen V.; Ulrichová J.; Vrba J. Sulfation modulates the cell uptake, antiradical activity and biological effects of flavonoids in vitro: An examination of quercetin, isoquercitrin and taxifolin. Bioorg. Med. Chem. 2015, 23 (17), 5402–5409. 10.1016/j.bmc.2015.07.055. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

