Tensile force impairs lip muscle regeneration under the regulation of interleukin-10
- PMID: 39351645
- PMCID: PMC11634486
- DOI: 10.1002/jcsm.13584
Tensile force impairs lip muscle regeneration under the regulation of interleukin-10
Abstract
Background: Orbicularis oris muscle, the crucial muscle in speaking, facial expression and aesthetics, is considered the driving force for optimal lip repair. Impaired muscle regeneration remains the main culprit for unsatisfactory surgical outcomes. However, there is a lack of study on how different surgical manipulations affect lip muscle regeneration, limiting efforts to seek effective interventions.
Methods: In this study, we established a rat lip surgery model where the orbicularis oris muscle was injured by manipulations including dissection, transection and stretch. The effect of each technique on muscle regeneration was examined by histological analysis of myogenesis and fibrogenesis. The impact of tensile force was further investigated by the in vitro application of mechanical strain on cultured myoblasts. Transcriptome profiling of muscle satellite cells from different surgical groups was performed to figure out the key factors mediating muscle fibrosis, followed by therapeutic intervention to improve muscle regeneration after lip surgeries.
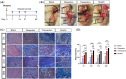
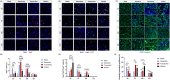
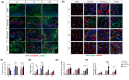
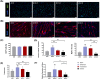
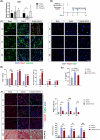
Results: Evaluation of lip muscle regeneration till 56 days after injury revealed that the stretch group resulted in the most severe muscle fibrosis (n = 6, fibrotic area 48.9% in the stretch group, P < 0.001, and 25.1% in the dissection group, P < 0.001). There was the lowest number of Pax7-positive nuclei at Days 3 and 7 in the stretch group (n = 6, P < 0.001, P < 0.001), indicating impaired satellite cell expansion. Myogenesis was impaired in both the transection and stretch groups, as evidenced by the delayed peak of centrally nucleated myofibers and embryonic MyHC. Meanwhile, the stretch group had the highest percentage of Pdgfra+ fibro-adipogenic progenitors infiltrated area at Days 3, 7 and 14 (n = 6, P = 0.003, P = 0.006, P = 0.037). Cultured rat lip muscle myoblasts exhibited impaired myotube formation and fusion capacity when exposed to a high magnitude (ε = 2688 μ strain) of mechanical strain (n = 3, P = 0.014, P = 0.023). RNA-seq analysis of satellite cells isolated from different surgical groups demonstrated that interleukin-10 was the key regulator in muscle fibrosis. Administration of recombinant human Wnt7a, which can inhibit the expression of interleukin-10 in cultured satellite cells (n = 3, P = 0.041), exerted an ameliorating effect on orbicularis oris muscle fibrosis after stretching injury in surgical lip repair.
Conclusions: Tensile force proved to be the most detrimental manoeuvre for post-operative lip muscle regeneration, despite its critical role in correcting lip and nose deformities. Adjunctive biotherapies to regulate the interleukin-10-mediated inflammatory process could facilitate lip muscle regeneration under conditions of high surgical tensile force.
Keywords: inflammation mediators; mesenchymal stromal cells; orofacial cleft; satellite cells, skeletal muscle; surgical procedures, operative.
© 2024 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
Xu Cheng, Jinfeng Dou, Jinggui Li, Yixuan Huang, Bing Shi and Jingtao Li declare that they have no conflict of interest.
Figures
References
-
- Park JA, Rho NK, Lee HI, Yeo IS, Koh KS, Song WC. Are there other muscle fibers on the orbicularis oris muscle in the upper lip? Plast Reconstr Surg 2022;150:1314e–1321e. - PubMed
-
- Noor RAM, Shah NSM, Zin AAM, Sulaiman WAW, Halim AS. Disoriented collagen fibers and disorganized, fibrotic orbicularis oris muscle fiber with mitochondrial myopathy in non‐syndromic cleft lip. Arch Oral Biol 2022;140:105448. - PubMed
-
- Martin SV, Van Eeden S, Swan MC. Secondary surgery techniques to optimise functional and aesthetic outcomes in orofacial clefting. Br Dent J 2023;234:899–905. - PubMed
-
- Denadai R, Chou PY, Pascasio DCG, Lo LJ. Modified unilateral incomplete cleft lip repair with primary nasal overcorrection: a muscle‐driven technique. Plast Reconstr Surg 2021;147:700–705. - PubMed
-
- Zhang C, Yao M, Low DW, Wu M, Shi B, Zheng Q, et al. Outcome comparisons of two different orbicularis oris muscle reconstruction techniques in patients with unilateral incomplete cleft lip. Plast Reconstr Surg 2023. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

