The enhancing effects of selenomethionine on harmine in attenuating pathological cardiac hypertrophy via glycolysis metabolism
- PMID: 39351650
- PMCID: PMC11443162
- DOI: 10.1111/jcmm.70124
The enhancing effects of selenomethionine on harmine in attenuating pathological cardiac hypertrophy via glycolysis metabolism
Abstract
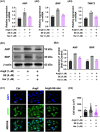
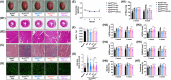
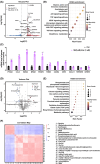
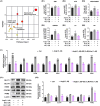
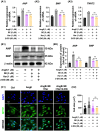
Pathological cardiac hypertrophy, a common feature in various cardiovascular diseases, can be more effectively managed through combination therapies using natural compounds. Harmine, a β-carboline alkaloid found in plants, possesses numerous pharmacological functions, including alleviating cardiac hypertrophy. Similarly, Selenomethionine (SE), a primary organic selenium source, has been shown to mitigate cardiac autophagy and alleviate injury. To explores the therapeutic potential of combining Harmine with SE to treat cardiac hypertrophy. The synergistic effects of SE and harmine against cardiac hypertrophy were assessed in vitro with angiotensin II (AngII)-induced hypertrophy and in vivo using a Myh6R404Q mouse model. Co-administration of SE and harmine significantly reduced hypertrophy-related markers, outperforming monotherapies. Transcriptomic and metabolic profiling revealed substantial alterations in key metabolic and signalling pathways, particularly those involved in energy metabolism. Notably, the combination therapy led to a marked reduction in the activity of key glycolytic enzymes. Importantly, the addition of the glycolysis inhibitor 2-deoxy-D-glucose (2-DG) did not further potentiate these effects, suggesting that the antihypertrophic action is predominantly mediated through glycolytic inhibition. These findings highlight the potential of SE and harmine as a promising combination therapy for the treatment of cardiac hypertrophy.
Keywords: cardiac hypertrophy; combination therapy; glycolysis metabolism; harmine; selenomethionine.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that this article content has no conflict of interest.
Figures


References
-
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358(13):1370‐1380. - PubMed
-
- Bernardo BC, Weeks KL, Pretorius L, Mcmullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128(1):191‐227. - PubMed
-
- Waki H, Park KW, Mitro N, et al. The small molecule harmine is an antidiabetic cell‐type‐specific regulator of PPARgamma expression. Cell Metab. 2007;5(5):357‐370. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

