Comparison of Ultrathin- Versus Thin-Strut Stents in Patients With High Bleeding Risk PCI: Results From the COMPARE 60/80 HBR Trial: An Open-Label, Randomized, Controlled Trial
- PMID: 39351676
- PMCID: PMC11472898
- DOI: 10.1161/CIRCINTERVENTIONS.123.014042
Comparison of Ultrathin- Versus Thin-Strut Stents in Patients With High Bleeding Risk PCI: Results From the COMPARE 60/80 HBR Trial: An Open-Label, Randomized, Controlled Trial
Abstract
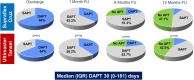
Background: No randomized data exist on ultrathin-strut stents in patients at high bleeding risk (HBR) undergoing an abbreviated dual antiplatelet therapy after coronary stenting. The aim of this study was to compare the safety and effectiveness of the ultrathin-strut biodegradable-polymer sirolimus-eluting Supraflex Cruz stent with the thin-strut biodegradable-polymer sirolimus-eluting Ultimaster Tansei stent in patients at HBR with abbreviated dual antiplatelet therapy after stenting.
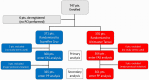
Methods: In the investigator-initiated, randomized, open-label COMPARE 60/80 HBR trial (Comparison of the Supraflex Cruz 60 Micron Stent Strut Versus the Ultimaster Tansei 80 Micron Stent Strut in HBR Percutaneous Coronary Intervention Population), 741 patients at HBR according to the Academic Research Consortium HBR criteria were randomized to receive either the ultrathin-strut biodegradable-polymer sirolimus-eluting Supraflex Cruz stent or thin-strut biodegradable-polymer sirolimus-eluting Ultimaster Tansei stent. Dual antiplatelet therapy was recommended according to the applicable guidelines and trial data for patients at HBR. The primary outcome was net adverse clinical events, the composite of cardiovascular death, myocardial infarction, target vessel revascularization, stroke, and major bleeding, and was powered for noninferiority with an absolute margin of 4.0% at 1-sided 2.5% alpha.
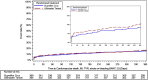
Results: Between September 2020 and August 2022, 371 patients were randomized to the ultrathin-strut biodegradable-polymer sirolimus-eluting Supraflex Cruz stent and 370 patients to the thin-strut biodegradable-polymer sirolimus-eluting Ultimaster Tansei stent at 11 sites in the Netherlands. At 1 year, the primary outcome was observed in 56 (15.4%) patients in the ultrathin-strut biodegradable-polymer sirolimus-eluting Supraflex Cruz stent group and 61 (17.1%) in the thin-strut biodegradable-polymer sirolimus-eluting Ultimaster Tansei stent group (risk difference, -1.65%; upper boundary of the 1-sided 95% CI, 3.74; P=0.02 for noninferiority at a 0.025 significance level and P=0.55 for 2-sided superiority at a 0.05 significance level).
Conclusions: Among patients at HBR with abbreviated dual antiplatelet therapy post-stenting, the use of an ultrathin-strut biodegradable-polymer sirolimus-eluting Supraflex Cruz stent was noninferior compared with the use of a thin-strut biodegradable-polymer sirolimus-eluting Ultimaster Tansei stent.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04500912.
Keywords: atherosclerosis; hemorrhage; percutaneous coronary intervention; platelet aggregation inhibitors; polymers; randomized controlled trials as topic; stents.
Conflict of interest statement
Dr Smits reports personal fees from Terumo and Abiomed, institutional grants and personal fees from Abbott Vascular and Microport, and institutional grants from SMT. Dr Hofma reports an institutional grant from Terumo. Dr Oemrawsingh reports speaker fees from Abbott Vascular and Philips. Dr Paradies reports speaker fees from Boston Scientific and Abbott Vascular and institutional grants from Abbott Vascular. The other authors report no conflicts.
Figures

References
-
- Koskinas KC, Chatzizisis YS, Antoniadis AP, Giannoglou GD. Role of endothelial shear stress in stent restenosis and thrombosis. pathophysiologic mechanisms and implications for clinical translation. J Am Coll Cardiol. 2012;59:1337–1349. doi: 10.1016/j.jacc.2011.10.903 - PubMed
-
- Madhavan MV, Howard JP, Naqvi A, Ben-Yehuda O, Redfors B, Prasad M, Shahim B, Leon MB, Bangalore S, Stone GW, et al. . Long-term follow-up after ultrathin vs. conventional 2nd-generation drug-eluting stents: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J. 2021;42:2643–2654. doi: 10.1093/eurheartj/ehab280 - PMC - PubMed
-
- Iglesias JF, Degrauwe S, Cimci M, Chatelain Q, Roffi M, Windecker S, Pilgrim T. Differential effects of newer-generation ultrathin-strut versus thicker-strut drug-eluting stents in chronic and acute coronary syndromes. JACC Cardiovasc Interv. 2021;14:2461–2473. doi: 10.1016/j.jcin.2021.09.028 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

