Recommendations for clinical implementation of blood-based biomarkers for Alzheimer's disease
- PMID: 39351838
- PMCID: PMC11567872
- DOI: 10.1002/alz.14184
Recommendations for clinical implementation of blood-based biomarkers for Alzheimer's disease
Abstract
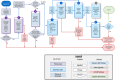
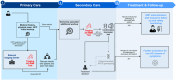
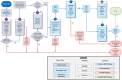
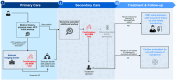
Blood-based biomarkers (BBM) for Alzheimer's disease (AD) are being increasingly used in clinical practice to support an AD diagnosis. In contrast to traditional diagnostic modalities, such as amyloid positron emission tomography and cerebrospinal fluid biomarkers, BBMs offer a more accessible and lower cost alternative for AD biomarker testing. Their unique scalability addresses the anticipated surge in demand for biomarker testing with the emergence of disease-modifying treatments (DMTs) that require confirmation of amyloid pathology. To facilitate the uptake of BBMs in clinical practice, The Global CEO Initiative on Alzheimer's Disease convened a BBM Workgroup to provide recommendations for two clinical implementational pathways for BBMs: one for current use for triaging and another for future use to confirm amyloid pathology. These pathways provide a standardized diagnostic approach with guidance on interpreting BBM test results. Integrating BBMs into clinical practice will simplify the diagnostic process and facilitate timely access to DMTs for eligible patients.
Keywords: Alzheimer's disease; amyloid; biomarker; blood‐based biomarkers; clinical implementation; clinical practice; cognitive impairment; disease‐modifying treatment; patient journey; primary care; secondary care.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
M.M.M. has consulted, or served on advisory boards, for Biogen, Eisai, LabCorp, Eli Lilly, Merck, Roche, Siemens Healthineers, and Sunbird Bio and receives grant support from the National Institute of Health, Department of Defense, and Alzheimer's Association. A.J. is an employee to ALZpath, Inc, and equity holder. P‐J. L. has consulted for Eli Lilly and reports research support from Biogen, Eisai, Eli Lilly, Genentech, and Incyte (to the institution). L.V. is a principal investigator for clinical trials sponsored by Biogen. O.H. has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche. In the past 2 years, O.H. has received consultancy/speaker fees from AC Immune, Amylyx, Alzpath, BioArctic, Biogen, Bristol Meyer Squibb, Cerveau, Eisai, Eli Lilly, Fujirebio, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens. A.S.K. received payments through organizational affiliations for grants, contracts, and consulting fees, honoraria, meeting support, travel support, in‐kind research/professional support over the last 36 months from the Alzheimer's Association, Acadia Pharmaceuticals, Alzheon, Biogen, Clinical Trials Alzheimer's Disease Conference, Davos Alzheimer's Consortium, Digicare Realized, Eisai, Eli Lilly, Embic, Relz Plc, High Lantern Group, International Neurodegenerative Disorders Research Center, and Serdi Publishing. S.E.S. has analyzed plasma biomarker data provided by C2N Diagnostics to Washington University; no personal or research funding was provided by C2N Diagnostics to S.E.S. S.E.S. has served on scientific advisory boards for Eisai. S.E.S. has an unpaid position on the Board of the Greater Missouri Alzheimer's Association. J.F.M. is a stockholder of Eli Lilly and Company. S.B. is an employee and stock owner at Hoffman–La Roche. E.S. is an employee and shareholder of Biogen. J.B.B. and M.M. are employees and shareholders of C2N Diagnostics. F.F.O. receives research support from FAPESP—The State of São Paulo Research Foundation. S.M. serves on the board of directors of Senscio Systems, Inc. and the scientific advisory board of AiCure Technologies, ALZPath and Boston Millennia Partners., and has received consulting and/or speaker fees from Biogen, C2N, Eisai, Novartis, Novo Nordisk, and Roche/Genentech. M.W.W. has served on Advisory Boards for Acumen Pharmaceutical, Alzheon, Inc., Cerecin, Merck Sharp & Dohme Corp., and NC Registry for Brain Health. M.W.W. also serves on the USC ACTC grant which receives funding from Eisai. M.W.W. has provided consulting to Boxer Captial, LLC, Cerecin, Inc., Clario, Dementia Society of Japan, Dolby Family Ventures, Eisai, Guidepoint, Health and Wellness Partners, Indiana University, LCN Consulting, MEDA Corp., Merck Sharp & Dohme Corp., NC Registry for Brain Health, Prova Education, T3D Therapeutics, University of Southern California (USC), and WebMD. M.W.W. holds stock options with Alzeca, Alzheon, Inc., ALZPath, Inc., and Anven. M.W.W. received support for research from the following funding sources: National Institutes of Health (NIH)/NINDS/National Institute on Aging (NIA), Department of Defense (DOD), California Department of Public Health (CDPH), University of Michigan, Siemens, Biogen, Hillblom Foundation, Alzheimer's Association, Johnson & Johnson, Kevin and Connie Shanahan, GE, VUmc, Australian Catholic University (HBI‐BHR), The Stroke Foundation, and the Veterans Administration. D.R.J., R.B., and Y.H.H. are employees of Eisai Inc. S. C. B. is an employee and minor shareholder of Eli Lilly and Company and has a patent for a method for the detection of neurological disease. M.A., J.W.A., A.R., J.T., D.W., J.W., J.R.D., D.H., K.A.P., E.S., G.V., D.Y., M.N.S., Z.M., and C.U‐M. declare no competing interests. Author disclosures are available in the Supporting Information.
Figures