Ribosome Quality Control mitigates the cytotoxicity of ribosome collisions induced by 5-Fluorouracil
- PMID: 39351862
- PMCID: PMC11551743
- DOI: 10.1093/nar/gkae849
Ribosome Quality Control mitigates the cytotoxicity of ribosome collisions induced by 5-Fluorouracil
Abstract
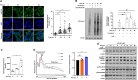
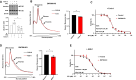
Ribosome quality control (RQC) resolves collided ribosomes, thus preventing their cytotoxic effects. The chemotherapeutic agent 5-Fluorouracil (5FU) is best known for its misincorporation into DNA and inhibition of thymidylate synthase. However, while a major determinant of 5FU's anticancer activity is its misincorporation into RNAs, the mechanisms by which cancer cells overcome the RNA-dependent 5FU toxicity remain ill-defined. Here, we report a role for RQC in mitigating the cytotoxic effects of 5FU. We show that 5FU treatment results in rapid induction of the mTOR signalling pathway, enhanced rate of mRNA translation initiation, and increased ribosome collisions. Consistently, a defective RQC exacerbates the 5FU-induced cell death, which is mitigated by blocking mTOR pathway or mRNA translation initiation. Furthermore, 5FU treatment enhances the expression of the key RQC factors ZNF598 and GIGYF2 via an mTOR-dependent post-translational mechanism. This adaptation likely mitigates the cytotoxic consequences of increased ribosome collisions upon 5FU treatment.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- Longley D.B., Harkin D.P., Johnston P.G.. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer. 2003; 3:330–338. - PubMed
-
- Doong S.L., Dolnick B.J.. 5-Fluorouracil substitution alters pre-mRNA splicing in vitro. J. Biol. Chem. 1988; 263:4467–4473. - PubMed
-
- Chen J.K., Merrick K.A., Kong Y.W., Izrael-Tomasevic A., Eng G., Handly E.D., Patterson J.C., Cannell I.G., Suarez-Lopez L., Hosios A.M.et al.. An RNA damage response network mediates the lethality of 5-FU in clinically relevant tumor types. 2023; bioRxiv doi:29 April 2023, preprint: not peer reviewed 10.1101/2023.04.28.538590. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

